Introduction:
Multiple myeloma (MM) is an incurable malignancy of B-cell lineage. Introduction of immunomodulatory drugs such as lenalidomide has significantly improved overall survival of patients with MM. Lenalidomide maintenance is currently the standard of care for maintaining remission in MM. However, second primary malignancies are known to arise in patients on lenalidomide though their pathogenesis is not known. We report a patient who developed B-lymphoblastic leukemia (B-ALL) while on lenalidomide, which went into spontaneous remission after stopping lenalidomide. Whole exome sequencing (WES) was performed to examine the mutational landscape and clonal evolution of the different malignant clones. We also aimed to postulate a mechanism for lenalidomide-induced ALL.
Case history and methods:
A 59-year-old female with history of rheumatoid arthritis was diagnosed with IgG-κ MM and treated with 8 cycles of bortezomib, Lenalidomide and dexamethasone followed by lenalidomide-maintenance (10 mg/day). At 36 months after initiation of treatment for MM, she developed lymphopenia and her bone marrow (BM) biopsy showed 38% leukemic B lymphoblasts. Lenalidomide was discontinued and a follow-up BM biopsy done 2 months later showed spontaneous complete remission of ALL, which was confirmed at 8 months. She remains in complete remission of ALL and MM without any specific treatment at 12 months after the diagnosis of ALL. BM aspirate samples at diagnosis of MM, on lenalidomide-maintenance with MM in remission, at diagnosis of ALL, and after stopping lenalidomide with MM and ALL in remission were used to perform WES with 2x150 bp reads and 100x coverage utilizing the Illumina Hiseq. The raw reads were mapped to reference genome (hg19) and compared with peripheral blood T-lymphocytes as germ line control. Variants were annotated using dbSNP, CLINVAR and COSMIC through Mutect. Clonal evolution was analyzed by SciClone. The study was approved by Institutional Review Board.
Results:
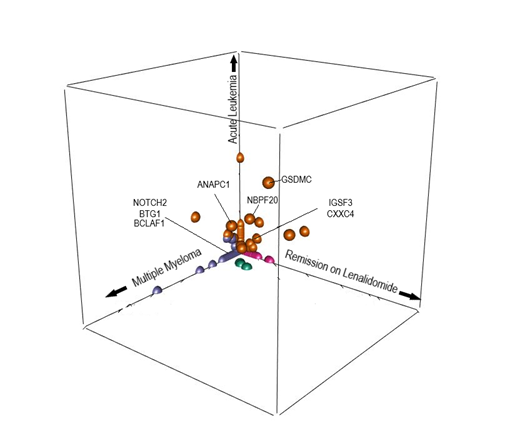
The burden of somatic mutations was significantly higher at diagnosis of MM when compared to the three other time points. Analysis of clonal architecture revealed the distinct clustering of mutations at specific time points (Figure). Most mutations detected at diagnosis of MM (e.g. NOTCH2, BTG1, BCLAF1) disappeared after treatment for MM. Similarly, most mutations detected only at diagnosis of ALL (e.g.PIK3CD, CDK16) became undetectable at spontaneous remission. Interestingly, clones with mutations of IGSF3 (immunoglobulin superfamily) and CXXC4 (Wnt signaling pathway) were detectable while the patient was on lenalidomide and at diagnosis of ALL but disappeared after stopping lenalidomide, which suggests that these clones gained pro-survival advantage from lenalidomide. Only a few mutations (GSDMC, NBPF20, ANAPC1) persisted in both MM and ALL stages. GSDMC is a gasdermin family member which may modulate function of MYC. NBPF20 has been described in relapsed pediatric ALL. ANAPC1 is a cell cycle gene whose transcription is regulated by Ikaros. Loss of repressor function of Ikaros was recently reported to deregulate ANAPC1 expression and cause mitotic progression of ALL in vitro. As lenalidomide is known to induce degradation of Ikaros, we hypothesize that lenalidomide may create a favorable selection pressure for B-cell clones harboring mutations in Ikaros-dependent genes.
Conclusions:
Clonal evolution analysis suggests that MM and ALL arose from different B-cell sub-clones, which was consistent with previous observation. However, there are a few shared mutations between MM and pre-B ALL, which may be responsible for leukemogenesis in our case. Lenalidomide may affect intracellular protein interactions to induce selection of rare B-cell clones evolving into secondary ALL. Also, our case demonstrated that simply stopping lenalidomide may lead to spontaneous and durable regression of ALL. Transcriptomic and proteomic analysis including Ikaros expression is required to further understand the mechanism of appearance of ALL and its regression after stopping lenalidomide.
Shlomchik:BlueSphere Bio: Other: Founder and Equity Interest.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal