Background. Previous studies have suggested a variation in the incidence of acute promyelocytic leukemia (APL) among geographic regions with relatively higher percentages within the Latin American population. We aimed to describe the population burden of pediatric APL (p-APL) in Brazil assessing the incidence rates according to a hospital-based and population-based cancer (PBCR) registries. We also explored mutations in genes of the RAS pathway and the association of polymorphisms in genes of glutathione S-transferases (GSTs) with outcome. Our goal was to provide insights into the distribution of clinical-demographic data and the molecular epidemiology characteristics associated with APL outcome.
Methods. One hundred and sixty-four p-APL cases (<19 years old) were identified from a dataset of a hospital-based registry based at a leukemia diagnostic central reference laboratory (2002-2018) and from 15 PBCR (2002-2009). Diagnostic criteria included morphological, immunophenotypic, and cytogenetic-molecular features. The PML-RARafusion gene was detected by FISH and/or RT-PCR. Additionally, mutations in FLT3 [D835 and internal tandem duplications (ITD)], KRAS, NRAS, and PTPN11 mutations were analyzed. We also evaluated the risk association of pharmacogenetically important GST deletion polymorphisms (GSTT1 and GSTM1) by multiplex-PCR, in a case-case analysis to test the effect of genetic susceptibility on overall survival (OS). Patients were treated with the inclusion of all-trans retinoic acid in chemotherapy with anthracyclines and cytarabine. Kaplan-Meier survival analysis was used to calculate the 5-year probabilities of OS (pOS), and estimated survival values were compared using the log-rank test. Cox proportional-hazard regression model estimated the hazard ratio (HR) and 95% confidence intervals (CI). All p-values were two-sided using significance level of 0.05.
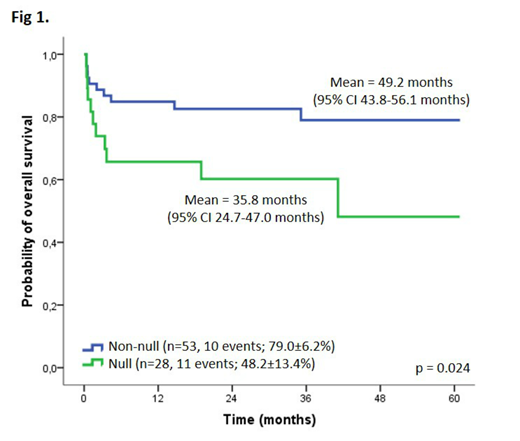
Results. From the hospital-based registry, APL patients represented 17.6% of the cohort (164 out of 933 acute myeloid leukemia cases), while in the PBCR, they represented 4.4% (35 out of 805 acute myeloid leukemia cases registered). The age-adjusted incidence rate was 0.32 per million persons during 2000-2009 based on PBCR. According to the hospital-based registry, we found similar distribution of patients among age ranges >2-10 and >10-21 years old (43.3% and 51.8%, respectively) and sex. RAS mutations were observed in 51.7% of APLs, including FLT3 (43.0%), NRAS (6.5%), and KRAS (2.2%). Variants in PTPN11 were silent amino acid substitutions (rs61736914; 4.9%). We observed a statistically significant association between FLT3 mutations and high white blood cell count at diagnosis (>10x109/L; 72.6%), low platelet count (<40×109/L; 83.0%), and the PML-RARa breakpoint cluster region 3 (90.5%). Death in the first ten days after diagnosis (early death) affected 17.5% (24/137) patients and these cases were excluded from the survival analysis. The mean of overall survival was 45.1 months (95% CI 40.1-50.1 months; pOS 66.9±5.8%). Univariate analysis did not show an association between variables and OS rates, except for APL patients carrying the GSTT1 polymorphism. GSTT1 null genotype conferred adverse prognosis compared to wild-type genotype (pOS 48.2±13.4% and 79.0±6.3%, p=0.024; hazard ratio 2.6, 95% CI 1.1-6.2, p=0.030; Figure 1).
Conclusions. APL represented 17.6% of acute myeloid leukemia in our cohort of Latino patients, which is a higher proportion compared to Northern European countries and the United States (5-10%). GSTT1 polymorphism modulated outcome, suggesting that lower enzyme activity may impact response to therapy. Decreasing early death and inclusion of GSTT1 polymorphism in therapeutic protocols for chemotherapy dose modulation may allow patients to reach the same overall survival observed in the United States (>70%).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal