BACKGROUND: Acute myeloid leukemia (AML) is the most common acute leukemia amongst adults which requires multiple phases and combinations of chemotherapeutic agents. Despite this complex regimen, most patients either fail to achieve remission or relapse. Triptolide, a diterpenoid triepoxide compound and Minnelide its water-soluble prodrug have shown significant efficacy in decreasing leukemic burden in preclinical animal models. In the current study, we evaluated the potential of Minnelide to prevent recurrence of AML via its effect on leukemic stem cells and in the bone marrow.
METHODS: To determine the effect of triptolide on the stemness of AML cells, two chemotherapy-resistant cell lines (THP-1 and KG-1) were treated overnight with triptolide at a dose of 2.5nM and 25nM. The colonies formed per well were subsequently measured. Stem cell markers (CD47, CD95, CD126 and TIM3) were also measured after treatment with various doses of triptolide (5nM, 10nM, 25nM). We also carried out in-vivo experiments in which luciferase-tagged THP-1 cells were intravenously injected into NSG mice. Positively implanted mice were then treated intraperitoneally with either saline or Minnelide (0.15mg/kg/day) for 30 days.
RESULTS: Triptolide treated cells had significantly reduced number of colonies per well in a colony formation assay, indicating decreased clonogenicity. In addition, treatment with triptolide reduced expressed of stem cell markers CD47 and CD126 at a concentration of 20nM (fold change of 0.3 and 0.55 for CD47 and fold change of 0.14 and 0.66 for CD126 for THP-1 and KG-1 respectively). In-vivo, Minnelide was able to successfully reduce tumor burden as evidenced by serial measurements of radiance (ROI) with IVIS. Furthermore, bone marrow histology of Minnelide treated mice resembled the bone marrow of non-diseased animals. Analysis with flow cytometry supported our findings showing a significant reduction of human CD45RA positive cells in the bone marrow of Minnelide treated mice.
CONCLUSION: Minnelide is not only successful in reducing tumor burden but it is potentially an effective therapy for preventing relapse of AML. This is evidenced by the reduction in stemness of AML cells treated with triptolide and through the reduction in tumor burden in the bone marrow of mice treated with low doses of Minnelide.
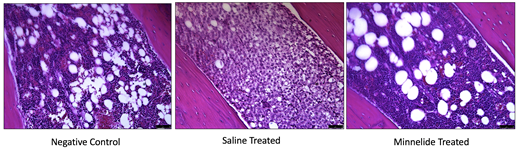
FIGURE 1: Bone marrow from Minnelide treated mice had a significantly reduced infiltration of myeloid cells compared to saline treated mice and even resembled negative controls.
Watts:Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Banerjee:Minneamrita Therapeutics LLC: Consultancy. Saluja:Minneamrita Therapeutics, LLC: Other: Co-founder and the Chief Scientific Officer.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal