Introduction
Venetoclax (VEN) is a selective BCL-2 inhibitor that has demonstrated activity against acute myeloid leukemia (AML) and has been shown to be effective when used in combination with hypomethylating agents (HMAs) or low-dose cytarabine (LDAC) for treatment-naïve, elderly AML patients unfit for intensive chemotherapy. Data on its use in the relapsed/refractory setting is limited.
Methods
A retrospective analysis was performed among 12 relapsed or refractory AML patients treated with VEN combination therapy at the University of California Los Angeles from 2018-2019. Seven patients received VEN in combination with azacitidine (75 mg/m2 x 7 days), 4 patients with decitabine (20 mg/m2 x 5 days), and 1 patient with low-dose cytarabine (20 mg/m2 x 10 days).
Results
The median patient age at time of VEN therapy was 58 years (range 41-79). Four patients (33.3%) had secondary AML. The majority (9 patients, 75.0%) had adverse cytogenetics. Three patients (25.0%) had received an allogeneic stem cell transplant prior to VEN therapy, and 5 patients (41.7%) had failed HMA therapy prior. Notable molecular mutations present were TP53 (4 patients, 33.3%), FLT3 (3 patients, 25.0%), and IDH2 (1 patient, 8.3%). Eight patients (66.7%) had grade 3 or greater neutropenia at time of VEN initiation, and 9 patients (75.0%) had grade 3 or greater thrombocytopenia. Four patients (33.3%) had a grade 3 or greater infection prior to VEN therapy.
Dosing of VEN was by physician discretion with a median starting dose of 150 mg (range 100-800) and a median maintenance dose of 450 mg (range 200-800). The median number of cycles of VEN combination therapy was 2 (range 1-5). Seven patients (58.3%) had decreased VEN dosage due to concomitant azole for antifungal prophylaxis. Four patients (33.3%) were on an additional small molecular inhibitor while receiving VEN therapy (sorafenib in 3 patients, ruxolitinib in 1 patient). The majority (10 patients, 83.3%) had an interruption in VEN dosing for the following reasons: bone marrow functional delay (7 patients), inability to tolerate oral pills (4 patients), infection (3 patients), and bleeding (2 patients).
The objective response rate (ORR) was 41.7% with 3 patients (25.0%) achieving complete remission with incomplete hematologic recovery (CRi) and 2 patients (16.7%) achieving partial remission (PR) (Table 1). Three patients (25.0%) experienced early death within 30 days due to the following: pneumonia (1 patient), multi-organ failure from infection and graft-versus-host disease (1 patient), and intracranial hemorrhage (1 patient). The median time to first and best response was 56 days (range 27-101) or after approximately 2 cycles of VEN combination therapy.
During VEN therapy, all patients (100%) had grade 3 or greater neutropenia and thrombocytopenia, and 10 patients (83.3%) had grade 3 or greater anemia. The nadir of most cytopenias occurred during cycle 1. Six patients (50.0%) developed a grade 3 or greater infection following VEN therapy, and 2 patients (16.7%) developed a grade 3 or greater intracranial hemorrhage. The only other notable grade 3 or greater side effects noted during VEN therapy were dizziness (1 patient, 8.3%) and diarrhea (1 patient, 8.3%).
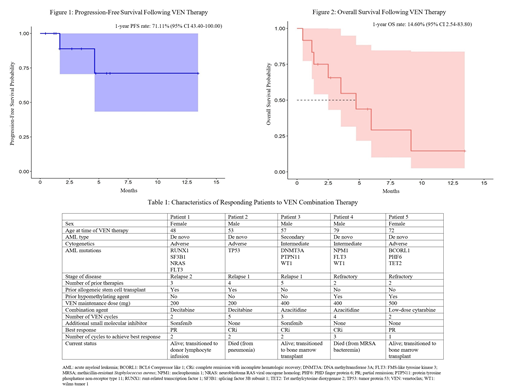
After a median follow-up time of 3.14 months (range 1.22-13.48), 2 patients (16.7%) progressed, and the 1-year progression-free survival (PFS) rate was 71.11% (95% CI 43.40-100.00) (Figure 1). Eight out of 12 patients died as a result of infection (6 patients, 50.0%), disease progression (1 patient, 8.3%), and bleeding (1 patient, 8.3%). The median overall survival (OS) was 4.74 months (range 1.18-9.15), and the 1-year OS rate was 14.60% (95% CI 2.54-83.80) (Figure 2). VEN was discontinued in all patients because of no response (5 patients, 41.7%), adverse effects (4 patients, 33.3%), transition to donor lymphocyte infusion (1 patient, 8.3%), or transition to allogeneic stem cell transplant (2 patients, 16.7%).
Conclusions
We present our institutional experience with VEN combination therapy for the treatment of relapsed/refractory AML with a particularly high-risk patient cohort, predominantly characterized by adverse genetic features and grade 3 cytopenias prior to start of therapy. Overall, the response rate was modest, but not inferior to that with conventional salvage chemotherapy. Adverse events were primarily due to pre-existing bone marrow failure, likely exacerbated by treatment.
Schiller:Agios: Research Funding, Speakers Bureau; Amgen: Other, Research Funding; Astellas: Research Funding; Biomed Valley Discoveries: Research Funding; Bristol Myer Squibb: Research Funding; Celgene: Research Funding, Speakers Bureau; Constellation Pharmaceutical: Research Funding; Daiichi Sankyo: Research Funding; Eli Lilly and Company: Research Funding; FujiFilm: Research Funding; Genzyme: Research Funding; Gilead: Research Funding; Incyte: Research Funding; J&J: Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Onconova: Research Funding; Pfizer Pharmaceuticals: Equity Ownership, Research Funding; Sangamo Therapeutics: Research Funding.
Venetoclax is a BCL-2 inhibitor approved for use in combination with azacitidine or decitabine or low-dose cytarabine for the treatment of newly-diagnosed acute myeloid leukemia in adults who are age 75 years or older, or who have comorbidities that preclude use of intensive induction chemotherapy. It does not currently have an approved use for the treatment of acute myeloid leukemia in the relapsed/refractory setting.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal