Background: Acute lymphoblastic leukemia (ALL) is the commonest cancer in children. The survival rates in pediatric ALL have improved to 80-90% in high-income countries, where the goal of current risk-adapted treatment protocols is to minimize treatment-related mortality. In contrast, cure/survival rates are still lower in low-income countries, due to combined effect of disease-related factors, high incidence of treatment abandonment, higher rates of relapse, & higher treatment-related mortality. The Berlin-Frankfurt-Münster (BFM) protocol versions are commonly used for treatment of pediatric ALL in many centers. There is no published data on treatment outcomes with ALL IC-BFM 2009 protocol in pediatric ALL in India.
Objective: The study aims to evaluate the treatment outcomes in Indian pediatric ALL/LBL patients treated with ALL IC-BFM 2009 protocol under severe resource-limited settings, in terms of morphological complete remission (CR), minimal residual disease (MRD) status, and disease-free survival.
Methods: Our study enrolled 25 newly diagnosed pediatric ALL/LBL patients (age ≤18 years) between February 2018 & June 2019. Induction chemotherapy was initiated as per ALL IC-BFM 2009 protocol after obtaining informed consent. Risk stratification was done as per protocol into standard risk (SR), intermediate risk (IR), and high risk (HR) groups, based on age, baseline leukocyte count, cytogenetics & molecular study findings, day 8 steroid response (peripheral blood absolute blast count), bone marrow MRD by flowcytometry on day 15, and bone marrow morphological response on days 15 & 33 of induction phase. Additional imaging was done to assess response in LBL patients. Central nervous system (CNS)-directed therapy with intrathecal chemotherapy was administered as per protocol. Post-induction therapy consisted of an augmented early intensification phase, risk-adapted consolidation therapy phase, a delayed intensification (reinduction) phase, & maintenance phase. Therapeutic cranial radiation (18 Gy) was administered only to patients with documented CNS leukemia.
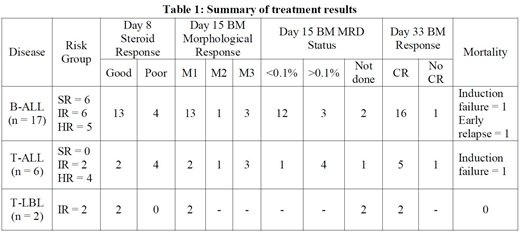
Results: Of the total 25 patients, 17 had B-ALL, six had T-ALL & two had T-LBL. Median age of patients was 9 years (2-18 years), with 11 boys & 14 girls. All the patients had BCR-ABL negative disease; three patients had t(12;21)(p13;q22)/ETV6-RUNX1. Seven patients (28%) had hyperleukocytosis (leukocyte count >1,00,000/µL) and two patients had CNS leukemia at presentation. Nine out of the 25 patients (36%) were in high risk group as per criteria. Eight patients (32%) had poor steroid response on day 8 of induction (PB blasts ≥ 1000/µL). Six patients had M3 marrow on day 15 (>25% blasts in marrow). Day 15 bone marrow MRD was <0.1% in 13 patients (52%), >0.1% in 7 patients (28%) and could not be done in 5 patients (including the two T-LBL patients). Twenty-three out of 25 patients (92%) achieved morphological CR (marrow blasts <5%) on day 33. There were two deaths in induction; both patients were not in CR as per day 33 bone marrow morphology criteria. One B-ALL patient in HR group died of early medullary relapse. The remaining 22 patients are in CR & doing well on follow up, and four of them have started maintenance phase therapy. The results are summarized in Table 1.
Conclusion: Our preliminary results with ALL IC-BFM 2009 treatment protocol in Indian pediatric ALL/LBL patients are encouraging, with 92% complete remission rate, 88% disease-free survival and manageable treatment-related toxicity in spite of severe resource constraints. Long-term follow up & enrollment of higher number of patients is planned.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal