BACKGROUND: After many decades of stagnation, several promising novel therapies have recently been approved for the management of patients (pts) with Acute Myeloid Leukemia (AML). Many of these target pts in specific clinical and molecular subsets such as midostaurin/gilteritinib for FLT3-mutated AML, liposomal daunorubicin-cytarabine for secondary AML, and fractionated gemtuzumab ozogamicin (GO) for good risk AML. To address the dismal outlook for elderly AML patients, we now have venetoclax or glasdegib combinations. Single agent FLT3 inhibitors (resulting in ~4 m improvement in overall survival (OS)) and IDH inhibitors (response rate 40%, doubled OS in responders) have shown to be superior to traditional approaches in the relapsed setting. Although these agents are exciting, a majority of pts with AML present with intermediate and high-risk disease and only a small subset will have actionable mutations. In order to determine the impact of these newer therapies on outcome for our pts, we investigated AML outcomes for pts diagnosed and treated since 2017 and compared these with survival for similar pts treated in the prior two years (y).
METHODS: We performed a retrospective chart review to identify newly diagnosed pts treated with chemotherapy over a 2y period (2015-17) prior to the first FDA approval of Midostaurin in April, 2017 (old AML era or group 1) and the 2y since approval (2017-19; new AML era or group 2). We reviewed charts of 138 AML pts meeting these criteria at our institution: 79 in group 1 and 59 in group 2. Demographics, disease-specific variables, as well as outcomes of interest (overall survival (OS), overall response rate (CR/CRi)) were collected on an IRB-approved protocol. Responses were defined according to the 2003 International Working Group (IWG) criteria. Demographics, baseline characteristics, and treatment responses were analyzed using descriptive statistics. Overall survival was estimated utilizing Kaplan-Meier (KM) survival analysis.
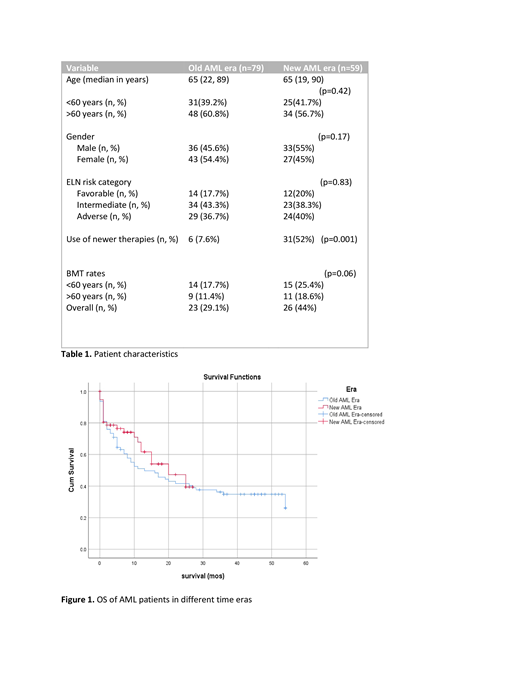
RESULTS: Clinical characteristics were comparable in both groups. Median age was 65y with slight differences in gender distribution (Table 1). ELN risk categories (Döhner, Blood. 2017) across the groups were similarly distributed; a majority of pts had intermediate and adverse risk characteristics. As expected, more pts received newer therapies in group 2. 6 pts (7.6%) in group 1 received new drugs (as part of clinical trials), while 31 (52%) received newer therapies in group 2. Newer therapies were mostly GO (28%), midostaurin (11%) and venetoclax (11%) with lesser percentages of the more specific targeted therapies. Median follow-up was 10m for group 1 and 8m for group 2. Median OS was 13m for group 1 and 20m for group 2, but the difference was not statistically significant (p=0.29) [Figure 1]. OS was significantly better in the subgroup of older AML pts (age ≥ 60y); median OS was 7m vs. 11m in groups 1 and 2 respectively (p=0.01).Rates of CR from induction therapy were comparable in both groups (68% in group 1 versus 65% in group 2). A larger number of pts (especially older pts) in group 2 went on to allogeneic bone marrow transplant (allo-HCT). This was perhaps due to increased recognition of performance status over age in selection of pts for allo-HCT and increased utilization of reduced intensity and non-myeloablative conditioning regimens.
CONCLUSIONS: Our results indicate a positive impact on survival for pts diagnosed in the era of novel therapies in a real world setting, specifically for pts presenting at older ages. A larger cohort analysis to further evaluate these findings is currently underway. The expansion of the therapeutic armamentarium as well as expanded transplant options is encouraging and offers new hope for pts diagnosed with AML
Griffiths:Onconova Therapeutics: Other: PI on a clinical trial; Partner Therapeutics: Consultancy; Astex Phramaceuticals/Otsuka Pharmaceuticals: Consultancy, Research Funding; Appelis Pharmaceuticals: Other: PI on a clinical trial; Onconova Therapeutics: Other: PI on a clinical trial; Appelis Pharmaceuticals: Other: PI on a clinical trial; Boston Scientific: Consultancy; Genentech, Inc.: Research Funding; Genentech, Inc.: Research Funding; New Link Genetics: Consultancy; Astex Phramaceuticals/Otsuka Pharmaceuticals: Consultancy, Research Funding; Celgene, Inc: Consultancy, Research Funding; Celgene, Inc: Consultancy, Research Funding; Abbvie, Inc.: Consultancy, PI on a clinical trial; Abbvie, Inc.: Consultancy; New Link Genetics: Consultancy; Persimmune: Consultancy; Persimmune: Consultancy; Boston Scientific: Consultancy; Novartis Inc.: Consultancy; Partner Therapeutics: Consultancy; Novartis Inc.: Consultancy. Wang:Kite: Other: Advisory role; Jazz: Other: Advisory role; Amgen: Other: Advisory role; Agios: Other: Advisory role; Abbvie: Other: Advisory role; Daiichi: Other: Advisory role; Stemline: Other: Advisory role, Speakers Bureau; Pfizer: Other: Advisory role, Speakers Bureau; celyad: Other: Advisory role; Astellas: Other: Advisory role, Speakers Bureau. Thota:Incyte, Inc.: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal