Introduction: On November 2018 the FDA approved the treatment of venetoclax together with hypomethylating agent (HMA) or low dose cytarabine (LDAC) for newly diagnosed AML patients who cannot tolerate intensive chemotherapy. There is limited data regarding the efficacy and safety of venetoclax in the setting of relapsed/refractory patients who received intensive chemotherapy for their disease.
Methods: In this study, data were collected retrospectively from Israeli medical centers where venetoclax was used as an advanced line of treatment after intensive chemotherapy. Venetoclax was given as a combination with HMA or LDAC or as monotherapy.
Results: 40 patients with AML were included in the study, 60% were Males. The median age at time of diagnosis was 67 years (range, 21-82). 27 (67.5%) patients had de-novo AML and 13 (32.5%) had secondary disease; 7 (17.5%) patients had a history of MDS and 6 (15%) of MPN. 37% of the patients had normal karyotype (20% of them had FLT3-ITD mutation), 23% had complex karyotype. Based on the 2017 ELN risk classification, 12.5% had favorable risk, 42.5% - intermediate and 45% had high risk.
At presentation, 87.5% of the patients received the '7+3' protocol as induction therapy but only 45% achieved a CR. Ultimately, 57.5% of the patients achieved a CR post consolidation/ salvage therapy. 42.5% underwent allogeneic hematopoietic cell transplant (HCT); 71% received myeloablative conditioning and the others reduced intensity.
The median time from diagnosis to starting venetoclax was 6 (1-64) months. Patients received venetoclax after a median of 2 (range, 1-4) prior lines of treatment (not including HCT). The median age at starting venetoclax was 68.5 years (range, 22-83).
The ECOG performance status at starting venetoclax was 0-1 in 67.5% of the patients, 2 in 22.5% and 3 in 10% of the patients.
The median daily dose of venetoclax was 400 mg (range: 100-800 mg). Venetoclax was given together with HMA (mostly azacitidine) in 62.5% of the patients and with LDAC in 22.5%. In 12.5% of the patients it was given as monotherapy and in 1 patient it was given with the FLAG-IDA regimen. The median number of cycles of venetoclax that were given so far is 2.75 (range: 0.5-14).
None of the patients experienced tumor lysis syndrome (TLS).
27.5% of the patients did not survive more than 2 months. Of the 29 patients who survived longer than 2 months, 76% achieved neutrophil recovery and 77% of them recovered also their platelet count. In 52% of those who survived more than 2 months, CR/CRi was confirmed by bone marrow (BM) examination. Taken together, 76% of the patients who survived more than 2 months achieved blood cell count recovery and in 68% of them it was confirmed as CR/CRi by BM examination.
At a median follow-up of 5.5 months, 35% of the patients are still alive and 57% of them are still on venetoclax. The leading cause of death (81%) was disease progression.
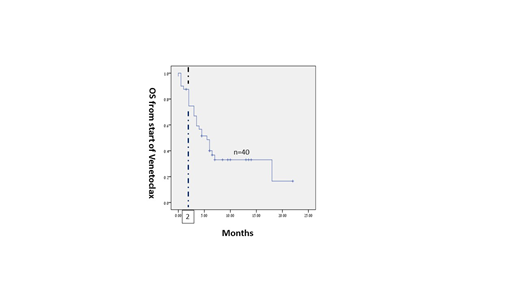
The median OS from starting venetoclax of all the patients and of those who survived more than 2 months was 5.5 and 6.5 months, respectively (Figure 1).
Conclusions: Venetoclax was given to high risk AML patients with relapsed/refractory disease after intensive chemotherapy and in around 40% of the patients also after HCT. The treatment was tolerable and no patient developed TLS. Among those who survived more than 2 months, the CR/CRi rate, or at least blood cell count recovery rate, was high (76%) and the median OS was 6.5 months. These retrospective data, albeit in a heterogeneous group of previously treated patients with AML, demonstrate that the combination of venetoclax with HMA or LDAC is safe and active also in AML patients with advanced disease. Its precise role for patients who relapse after intensive chemotherapy needs to be evaluated in a prospective clinical trial.
Wolach:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Speaker; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Speaker. Rowe:BioSight: Consultancy.
Venetoclax for relapsed/ refractory AML
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal