Background: Mutations of the gene encoding Fms Related Tyrosine Kinase 3 (FLT3), at the juxta-membrane level (ITD), represent the most common lesions found in Acute Myeloid Leukemia (AML), identifying a subgroup of patients (pts) with unfavorable prognosis. FLT3-ITD mutations are considered an unreliable tool for measurable residual disease (MRD) monitoring, due to their intraclonal heterogeneity and instability during the course of disease. Instead, multiparametric flow cytometry (MFC) may represent an alternative to monitor MRD in this molecular subset. In fact, through the recognition and monitoring of leukemia associated immunophenotypes, MFC is applicable to > 90% of AML patients with a sensitivity of 10-4.
Aims: The aim of our study was to investigate the reliability of MFC in MRD assessment of 72 FLT3-ITD positive pts whose treatment allocation was prospectively decided according to the genetic/cytogenetic profile at diagnosis and post consolidation MRD. FLT3-ITD pts were to receive, after induction and consolidation, allogeneic stem cell transplant (ASCT), whatever the source of stem cells. In this subgroup analysis, we investigated if FLT3-ITD mutated pts have a different propensity to achieve high quality (e.g. MRD negative) complete remission as compared to FLT3 wildtype ones. Furthermore, we seek for a correlation between different levels of MRD and overall (OS) and disease-free survival (DFS).
Methods: We included in the analysis 72 pts with de novo AML carrying FLT3-ITD mutations whose MRD assessment at the post-consolidation timepoint was available. Pts were defined as MRD-negative, when obtaining a residual leukemic cells count below the threshold of 3.5x10-4 (0.035%). MRD positive pts (with MRD ≥ 3.5x10-4 RLC) were stratified into 3 classes according to the levels of MRD (0.035%-0.1%; >0.1%-1%; >1%). We compared the MRD status and clinical outcome with a matched group of FLT3 wildtype AML (n = 203) treated in the same protocol.
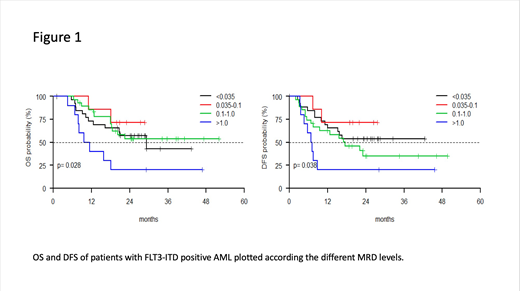
Results: Overall median age was 49 (range 18-60.9). The 2 cohorts were balanced in terms of age and sex distribution. In the FLT3-ITD group, 80/126 (64%) cases carried a concomitant NPM1 mutation vs 107/374 (28.6%) of FLT3 wildtype ones (p <0.001). Furthermore, FLT3 mutated pts had a median WBC count of 35x109/L vs 9.5x109/L of those FLT3 wildtype (p < 0.001). MRD determination after consolidation cycle was available in 72/126 FLT3-ITD pts (57%) and in 203/374 FLT3 wildtypeones (54.3%), respectively. After having received induction and consolidation course, 47/72 FLT3-ITD pts (65,2%) were submitted to allogenic stem cells transplantation (ASCT). At the post-consolidation time-point, MRD negativity rate was significantly lower in FTL3-ITD pts (27/72, 37.5%) as compared to those FLT3 wildtype (94/203, 46.3%). Furthermore, 38/72 (52.8%) and 10/72 (13.9%) FLT3-ITD pts had a level of MRD > 0.1% and > 1%, respectively as compared to 65/203 (33.0%) and 15/203 (7.4%) of FLT3 wildtypeones, respectively (p=0.017). When considering the different MRD stratification levels of FLT3-ITD pts, OS probability at 24 months was 57.2% (27 pts), 71.4% (7 pts), 53.6% (28 pts) and 20% (10 pts), for the MRD categories <0.035%, 0.035%-0.1%, >0.1%-1%, >1%, respectively (p=0.028). DFS probability at 24 months was 53.8% (27 pts), 71.4% (7 pts), 34.9% (27 pts) and 20% (10 pts), for the MRD categories <0.035%, 0.035%-0.1%, >0.1%-1%, >1%, respectively (p=0.038).
Summary/Conclusion: We demonstrated that MRD determination by MFC is a reliable tool to assess remission quality and prognosis in FLT-ITD positive patients. This subpopulation shows a lower propensity to obtain a MRD negative CR, with the majority of pts maintaining an amount of MRD > 0.1% after standard treatment. Even though most of these pts were addressed to ASCT, post-consolidation MRD maintained its negative impact on OS and DFS, particularly for those pts with MRD >1%. In the attempt to improve the quality of response, prevent leukemia recurrence and pursue a durable remission, delivery of FLT3 inhibitors as a maintenance after transplant may represent a promising option.
Venditti:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Astellas: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees. Buccisano:Janssen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal