Malignant hematopoietic cells of myelodysplastic syndromes (MDS)/chronic myelomonocytic leukemias (CMML) and acute myeloid leukemias (AML) may be particularly vulnerable to inhibition of poly(ADP ribose) polymerase 1/2 (PARP1/2) and apurinic/apyrimidinic endonuclease 1 (APE1). PARP1/2 and APE1 are critical enzymes involved in single-strand break repair and base excision repair, respectively. Here, we investigated the cytotoxic efficacy of talazoparib and APE1 inhibitor III, inhibitors of PARP1/2 and APE1, as single-agents, combined with decitabine and combined with each other in CD34+ MDS/CMML cells and in CD34+ or CD34- AML cells in comparison to healthy CD34+ donor cells.
The surviving fraction of CD34+ MDS/CMML cells (n = 8; 4 MDS and 4 CMML), CD34+ or CD34- AML cells (n = 18) and healthy CD34+ donor cells (n = 8) was analyzed using the CellTiter-Glo luminescent cell viability assay (Promega, Southampton, UK). Cell proliferation of untreated MDS/CMML and AML cells was determined by trypan blue exclusion assay (Merck, Darmstadt, Germany). PARP1/APE1 mRNA expression was evaluated using validated primer sets for PARP1 (Hs_PARP1_1_SG QuantiTect Primer Assay, NM_001618) and APE1 (Hs_APEX1_1_SG QuantiTect Primer Assay, ENST00000216714) (Qiagen, Hilden, Germany). Immunofluorescence microscopy of γH2AX foci was performed using a JBW301-derived mouse monoclonal anti-γH2AX antibody (Merck).
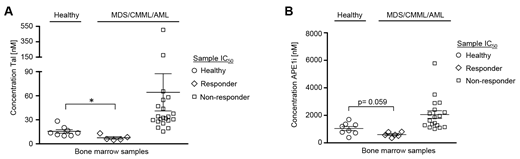
Talazoparib and APE1 inhibitor III demonstrated critical anti-leukemic efficacy as single-agents in about 19-25% of MDS/CMML/AML cell samples (Figure 1A and B). Low doses of talazoparib and APE1 inhibitor III further increased the cytotoxic efficacy of decitabine in about 78-86% of MDS/CMML/AML cell samples. Moreover, low doses of APE1 inhibitor III increased the cytotoxic efficacy of talazoparib in about 68% of MDS/CMML/AML cell samples.
In summary, talazoparib and APE1 inhibitor III demonstrated substantial anti-leukemic efficacy as single-agents, in combination with decitabine and combined with each other. Hence, our findings support further investigation of these agents in sophisticated clinical trials.
Figure 1 Cytotoxic efficacy of talazoparib and APE1 inhibitor III in healthy CD34+ donor cells, in CD34+ myelodysplastic syndrome (MDS)/chronic myelomonocytic leukemia (CMML) cells and in CD34+ or CD34- acute myeloid leukemia (AML) cells after 3 days of treatment. (A) The mean IC50 of talazoparib was significantly lower (*p = 0.016) in 1 MDS (MDS#2), 1 CMML (CMML#2) and 3 AML cell samples (AML#1, AML#2, AML#3) as compared to 8 healthy donor cell samples. (B) The mean IC50 of APE1 inhibitor III was substantially lower (p = 0.059) in 1 MDS (MDS#2) and 5 AML cell samples (AML#1, AML#2, AML#3, AML#6, AML#12) as compared to 8 healthy donor cell samples.
Fabarius:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal