Acute myeloid leukemia (AML) is a highly heterogeneous disease with poor clinical prognosis, especially to cytogenetically normal AML(CN-AML)patients, which belong to moderate prognosis group. The clinical outcomes of them are not consistent. Therefore, further exploration of novel biomarkers and development of new anti-tumor drugs is urgently needed. Recently, accumulating evidence have emerged and demonstrated that branched-chain amino acids (BCAA) are essential for tumor growth and proliferation. Branched-chain aminotransferase 1(BCAT1), a BCAA metabolic enzyme, which correlates with cancer aggression, not only that, it has been reported that overexpression of BCAT1in leukemia cells decreased intracellular αKG levels, showed the characteristics of stem cells and displayed a phenotype like cases carrying IDH mutations. By limiting intracellular αKG, BCAT1high expression AML cells prone to cause an increase in DNA damage probably via suppressing homologous recombination.
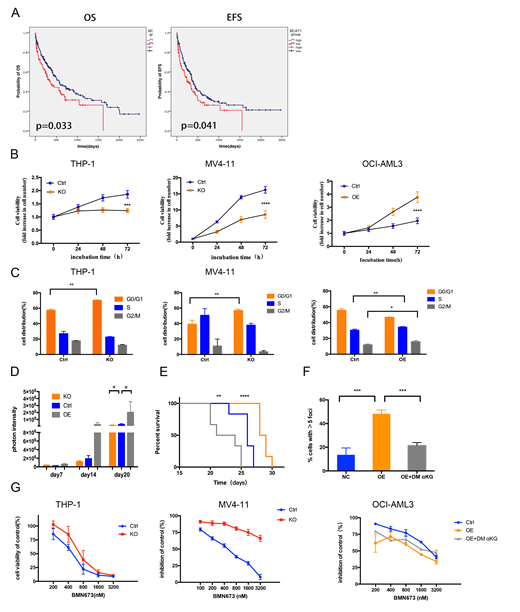
We firstly analyzed the prognostic significance of BCAT1expression in CN-AML patients. Collectively, we found that patients with high levels of BCAT1had poor prognosis, the median overall survival time is shorter in BCAT1high group (356 days versus 570 days)(Figure A). Next, we performed gene knockout in THP-1 and MV4-11 cells with relatively high expression of BCAT1and gene overexpressing in the relatively low expression cell line OCI-AML3. In summary, BCAT1overexpression contributed to cell growth, whereas suppression of BCAT1markedly limited cell proliferation and colony-forming ability by blocking cell circle at G0/G1 phase(Figure B and C).The mice model we conducted further validated the role of BCAT1expression in vitro. Notably, we found that knockout and overexpression of BCAT1can respectively reduce and enhance the tumor burden(Figure D)and effects the time of survival(Figure E).
Because both BCAT1high expression and IDH mutation could decrease αKG levels and suppress αKG dependent dioxygenases, it is tempting to speculate that there is a possibility of a "BRCAness" phenotype when BCAT1is overexpressed.We indeed observed a rise in the base line level of DNA damage marker γ-H2AX in BCAT1overexpressing cell line(Figure F). In addition, the sensitivity of cells to cisplatin is also positively correlated with BCAT1expression levels, the IC50 of BCAT1overexpressing cell line OCI-AML3 is much lower than control. Consistently, knocking out of BCAT1decreased sensitivity to cisplatin. So we further tested the relationship between PARPi talazoparib(BMN 673) and BCAT1expression, Use a gain of function approach we increased susceptibility to PARPi, together with the loss of function data, these results strongly suggest that BCAT1promotes cell sensitivity to PARPi(Figure G). We believe that the combination of high expression of BCAT1and PARPi produces synthetic lethality. In addition, the ability to trap PARP1 of PARPi may produce unacceptable toxicity when combined with conventional doses of cytotoxic chemotherapies. So, we next combined PARP inhibitors with DNA-damaging agent daunorubicin, the synergistic effect is promising on BCAT1overexpressed OCI-AML3 cell especially at high dose, however, the synergistic effect of the control group is weak, sometimes even manifested as antagonistic.Consistent with previous reports, IDH1/2mutAML is vulnerable to PARP inhibition as monotherapy, but especially when combined with daunorubicin treatment. These results also confirmed our hypothesis that BCAT1overexpression mimics the phenotype of IDHmutcells, not only as DNA hypermethylation, but also leads to increased DNA damage levels in cells, increasing sensitivity to PARP inhibitor and DNA-damaging drugs. Through qPCR and WB assays, we found the same trend: DNA damage response-related protein ATM was down-regulated after BCAT1overexpression, which preliminarily explained why DNA damage was increased after BCAT1overexpression. After exogenous supplementation with the aKG analog DM-aKG, the enhancement of DNA damage caused by overexpression of BCAT1can be reversed.
In summary, this study indicated that CN-AML patients with high BCAT1expression had poor prognosis. BCAT1plays the role as oncogene, can induce DNA damage that renders AML cells sensitive to PARP inhibition and DNA damage agents, and can be used as a novel therapeutic option for AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal