Background
Immunoglobulin (IVIG) is used to treat autoimmune conditions, but there are reports of brisk hemolysis within 48 hours (hrs) of treatment due to anti-A isohemogglutinins(1). Despite these reports, hemolysis remains an unrecognized side effect of IVIG.
Methods
We presented a series of 3 cases of IVIG-induced hemolysis in patients with autoimmune neurological disorders. In the investigative phase, we traced the cases to a common IVIG lot number. The sample was tested to determine the anti-A titer levels.
Case Studies (See Table 1)
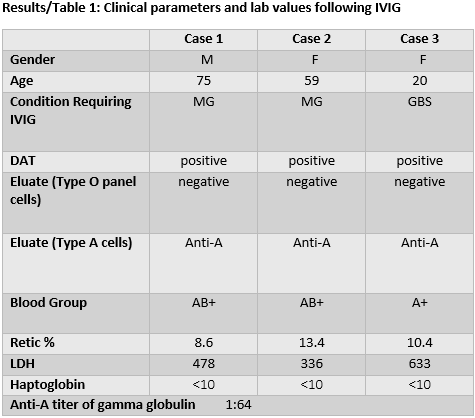
Case 1
75-year-old man presented with SOB and dysarthria from myasthenia gravis (MG). He received IVIG for 4 days. He developed a hemolytic anemia with 3 g drop in hemoglobin (Hb) 48 hours later. He needed a pRBC transfusion and folic acid.
Case 2
59-year-old female with history of MG treated with IVIG at another hospital until 3 months earlier in crisis, with SOB and dysphagia. She received IVIG for 5 days and rituximab. She improved and was discharged, but returned to the ER 7 days later with SOB. Her Hb fell to 8.0 g/dL from 13 g/dL on last admission. She required a pRBC transfusion, folic acid, and vitamin B12 with improvement of SOB.
Case 3
20-year-old female admitted for lower extremity weakness, diagnosed with presumed syndrome (GBS). She received IVIG for 4 days. On the 5th day, her Hb fell from 15 g/dL to 9 g/dL. She began prednisone, folic acid, and vitamin B12 with improvement in her Hb.
Conclusions
Although acute hemolysis is well described in the literature, it is under recognized, as exemplified by the first two cases. Their initial SOB was due to MG, so when SOB recurred, they were misdiagnosed with recurrent MG. A hemolytic anemia was later suspected, and a work up revealed a positive DAT. The initial eluate was negative against type O panel cells, suggesting a drug related hemolysis. It was only when the eluate was tested against type A cells that the etiology became clear. The third patient's hemolytic reaction was then rapidly identified. These cases remind us to consider IVIG induced anti-A hemolysis in patients who are blood type A and AB, and to evaluate the eluate against the appropriate reagent cells. These patients should receive specific IVIG that is low in anti-A isohemagglutinins. Since the second patient did not hemolyze from earlier exposure to IVIG, she likely received a low titer product.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal