Background: Congenital fibrinogen deficiencies (CFD), including afibrinogenemia and hypofibrinogenemia, are associated with severe and/or frequent bleeding episodes (BEs) that can occur spontaneously or following trauma. Human fibrinogen concentrate (HFC) has been shown to rapidly restore hemostasis in the event of such bleeding. The safety and efficacy of a new highly purified HFC have been demonstrated in interventional clinical studies; however, real-world evidence collected from routine clinical practice is needed to corroborate these data.
Aims: The FORMA-07 study will collect real-world evidence from clinical practice concerning the safety and efficacy of a new HFC in the treatment of CFD. This highly purified, plasma-derived, lyophilized HFC undergoes two dedicated virus inactivation/removal steps during production.
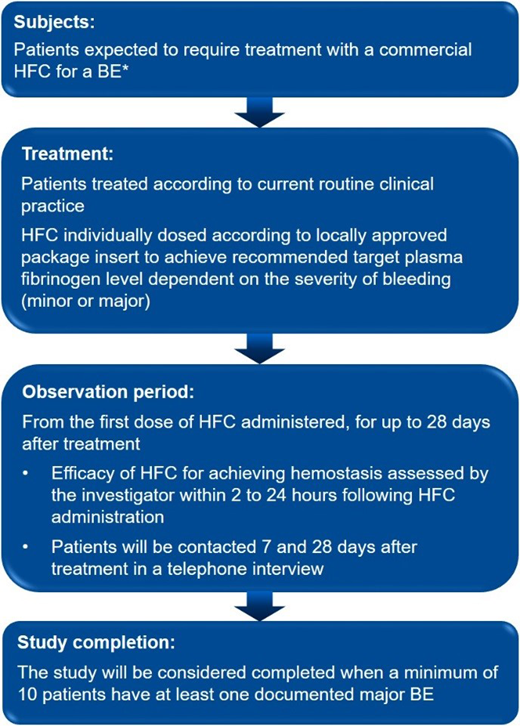
Methods: The FORMA-07 study is a post-marketing, multicenter observational study in adults and adolescents (≥12 years) with congenital afibrinogenemia or hypofibrinogenemia who are expected to require on-demand treatment with HFC for BEs. The study will examine treatment with a highly purified, plasma-derived, double virus inactivated/eliminated, lyophilized HFC (Octapharma). This Octapharma-sponsored study will seek approval from independent ethics committees prior to study start. Informed consent will be obtained from all subjects. Exclusion criteria include: other bleeding disorders, including acquired fibrinogen deficiency and dysfibrinogenemia; anti-fibrinogen inhibitors; participation in concurrent clinical studies. The study outline is shown in Figure 1.
The primary endpoint is the incidence of thromboembolic adverse drug reactions (ADRs) in patients receiving HFC for on-demand treatment of BEs. Secondary endpoints include the hemostatic efficacy of HFC for treatment of all BEs recorded in the study (with focus on major BEs), assessed by the investigator using a 4-point objective scale within 2-24 hours following treatment. Safety endpoints include ADRs, serious ADRs, and ADRs of special interest (i.e., thromboembolic events and allergic reactions, including anaphylaxis).
The planned study duration is up to 6 years and the study aims to enroll a minimum of 25 patients treated with HFC, to describe 105 BEs, including a minimum of 10 major BEs in 10 patients. During the observation period, enrolled patients may be treated for any BEs.
Results: Results will be monitored on an ongoing basis and the study is expected to end Q2 2025.
Conclusions: This study will collect real-world evidence for the safety and efficacy of HFC during routine clinical use in the treatment of bleeding in patients with congenital fibrinogen deficiency.
Schwartz:Octapharma: Employment. Peyvandi:Alnylam: Honoraria; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Grifols: Honoraria; Kedrion: Honoraria; Octapharma: Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Solomon:Octapharma: Employment. Knaub:Octapharma: Employment. Toth:Octapharma: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal