Background: Heparin-induced thrombocytopenia (HIT) is an immune mediated, pro-thrombotic disorder associated with exposure to heparin and a substantial number of patients develop thrombosis (HITT) in the venous, arterial, or microvascular system. Treatment includes cessation of heparin and starting a non-heparin anticoagulant. Patients with cancer are already at high risk of venous thromboembolism (VTE) as well as recurrent VTE despite anticoagulant therapy and are also at higher risk of bleeding compared with patients without cancer. Consequently, cancer patients may not share similar outcomes as patients without cancer in the setting of HIT. We conducted a single-centre, retrospective study to evaluate baseline characteristics, treatments and outcomes in HIT patients with and without cancer.
Methods: Medical records of all patients seen at our tertiary centre between November 1, 2006 and December 31, 2016 who tested positive for HIT antibodies and had a 4T score of 4 or higher were reviewed. Patients with cancer were defined as those who had any evidence of cancer, including myeloproliferative neoplasm (MPN), and/or receiving cancer treatment within 6 months prior to HIT diagnosis. Details of treatments and outcomes were captured up to 6 months after start of HIT treatment. Comparative statistics was performed between the cancer and non-cancer cohorts.
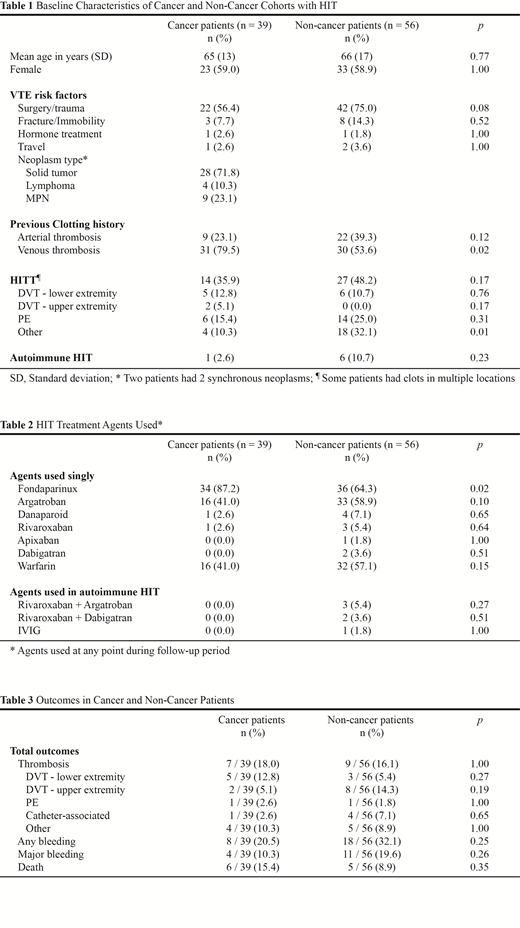
Results: We identified 95 patients with confirmed HIT, of whom 39 (41%) had cancer and 41 (43%) had HITT as the index event. The mean age was 65 years (standard deviation 16) and 59% were female. Thirty (77%) cancer patients had at least 3 months of available records and 26 (67%) had at least 6 months, while 37 (66%) non-cancer patients had at least 3 months of available records and 27 (48%) had at least 6 months. Baseline demographics including cancer types are summarized in Table 1. The most common malignancy was polycythemia vera (PV), with those with MPN (7 PV, 2 essential thrombocythemia) representing 23% of the patients with cancer. Cancer patients were more likely to have a history of thromboembolic events prior to index heparin exposure and HIT diagnosis (79.5% vs. 53.6%, p=0.02) than those without cancer. Among patients with HITT, the two groups had similar incidences of pulmonary embolism and/or deep vein thrombosis, although a higher proportion of the non-cancer group had clots in other non-classic locations (32.1% vs. 10.3%, p=0.01) such as splanchnic thrombosis. A variety of non-heparin agents were used, including direct oral anticoagulants (Table 2), with most patients receiving either fondaparinux or argatroban followed by warfarin. The cancer group received fondaparinux more often than the non-cancer group (87.2% vs. 64.3%, p=0.02). In those alive with at least 6 months of follow-up, the median duration of non-heparin anticoagulation was 180 days for both cancer patients and non-cancer patients. During follow-up, 16 (17%) patients had a thrombotic event, 15 (16%) had major bleeding and 11 (12%) died among the 95 patients with HIT. The rates of subsequent thrombosis, bleeding events, and death were similar between the two cohorts over the 6-month follow-up period (Table 3). None of the deaths were from thrombotic or bleeding events but the cause of death for one patient with cancer was unknown.
Conclusion: Patient outcomes following a diagnosis of HIT appear similar between patients with and without cancer, with high rates of subsequent thrombosis and major bleeding. Patients with MPN might have a higher risk of HIT. Further studies are warranted to confirm these findings and determine if direct oral anticoagulants might be efficacious and safe in patients with HIT.
Lee:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria; LEO Pharma: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.
Direct oral anticoagulants and fondaparinux were used as non-heparin anticoagulants for the treatment of heparin induced thrombocytopenia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal