Introduction: Efmoroctocog alfa (Elocta®) is a recombinant coagulation FVIII-Fc (rFVIIIFc), a fully recombinant fusion protein produced in human embryonic kidney cells, with an extended half-life used for the treatment and prevention of bleeding in patients with severe hemophilia A. Using rFVIIIFc for the treatment of severe hemophilia A patients received the approval of reimbursement in Spain at the end of 2016. Therefore, there are no many comparative data published about real life use of rFVIIIFc.
Objective: This work aims to describe characteristics of the treatment of severe hemophilia A patients with rFVIIIFc and to compare its results with those previously obtained employing other FVIII products.
Methods: This was an open-label non-interventional retrospective study reviewing patient characteristics and treatment outcomes before and after the use of rFVIIIFc.
The La Paz University Hospital Ethics Committee approved the experimental protocol.
Patients with severe hemophilia A without inhibitors being treated with rFVIIIFc since at least six months before study approval by Ethics Committee were included.
The following data were collected for patients included in the study: dose (IU/kg) and prophylaxis treatment regimen, number of spontaneous and traumatic bleedings, annual bleeding rate (ABR) and FVIII trough level.
The statistical analysis on the variables listed above comparing before and after rFVIIIFc usage was performed by the Biostatistics Unit of La Paz University Hospital with the statistical package SPSS v.18.0 (SPSS Inc., Chicago, IL, USA).
Results: Twenty two severe hemophilia A patients (median age: 20 years old, ranging from 6 to 63 years) on prophylaxis with rFVIIIFc were considered to be included in this study, but two were excluded due to lack of data.
Median follow-up period was 14 months (ranging from 6 to 28 months). Nineteen severe hemophilia A patients have been previously treated with rFVIII (two of them with other extended half-life product) and one with plasma-derived FVIII.
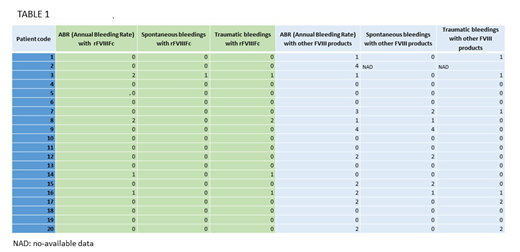
Eight of the ten severe hemophilia A patients who presented an ABR greater than 0 with previous treatments reduced their ABR when treated with rFVIIIFc (Table 1). Among those patients with an ABR=0 with previously used FVIII products, only one increased to an ABR=1 when treated with Elocta® due to a traumatic bleeding. Table 1 shows ABR across all patients before and after rFVIIIFc.
There was no difference in dose per injection between other FVIII products and rFVIIIFc (median dose for patients treated with other FVIII products: 46.0 IU/kg, ranging from 26 to 65 IU/kg; median dose for patients treated with rFVIIIFc: 46.5 IU/kg, ranging from 26 to 65 IU/kg). Nevertheless, a reduction was observed in administration frequency. Among the twelve patients who received treatment with other FVIII products every 48 hours, eleven came to receive rFVIIIFc 3 times a week and the one previously receiving a plasma-derived FVIII, to twice a week. Five of the patients receiving treatment 3 times a week reduced its frequency to twice per week. Three patients maintained the same schedule of administration. To note, one of the two patients receiving another prolonged half-life product maintained the schedule of treatment and the other reduced its frequency from every 48 hours to 3 times a week.
FVIII trough level in plasma (% of FVIII), expressed as median (25th-75th percentile), was 1.1 (0.1-4.0) for rFVIIIFc treatment and 0.2 (0.0-1.9) for other FVIII products (p=0.06).
Conclusions:
85% of the severe hemophilia A patients from our cohort reduced the weekly dose administration after beginning treatment with rFVIIIFc.
Most of the patients increased plasma trough level of FVIII with rFVIIIFc.
45% of patients reduced and 40% kept their ABR=0 when they changed rFVIIIFc.
These data suggest that treatment with rFVIIIFc gives a higher protection to severe hemophilia A patients. However, further research with larger sample size is required to investigate this.
This work was supported by SOBI. NB holds a tenure track grant from FIS-FONDOS FEDER (CP14/00024).
Álvarez Roman:Takeda: Research Funding; Amgen: Consultancy, Speakers Bureau; NovoNordisk: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau; Sobi: Consultancy, Speakers Bureau. Fernandez-Bello:Novartis, Pfizer, ROCHE, Stago: Speakers Bureau. Martín:SOBI: Research Funding; Novartis, Pfizer, ROCHE, Novo Nordisk: Speakers Bureau. Rivas Pollmar:Novartis, Pfizer, ROCHE, Novo Nordisk: Speakers Bureau; SOBI: Research Funding. García Barcenilla:Bayer, Pfizer, Takeda, Novartis: Speakers Bureau; SOBI: Research Funding. Canales:SOBI: Research Funding; iQone: Honoraria; Karyopharm: Honoraria; Novartis: Honoraria; Takeda: Speakers Bureau; Gilead: Honoraria; Celgene: Honoraria; Janssen: Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Honoraria, Speakers Bureau; Sandoz: Honoraria. Butta:Roche, Pfizer: Speakers Bureau; Novartis: Consultancy. Jimenez-Yuste:Bayer, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, Shire: Consultancy, Honoraria, Other: reimbursement for attending symposia/congresses , Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal