Introduction
Immune thrombocytopenia (ITP) is a disease characterized by low platelet count and increased risk of bleeding. However, there has been an increased focus on the risk of thromboembolism particularly venous thromboembolism (VTE) associated with ITP itself and with some of its therapies. Epidemiological studies have shown a doubling in the risk of VTE in ITP patients as compared to the general population. VTE has been reported with various ITP therapies including splenectomy, steroids, IVIG and thrombopoietin receptor agonists (TPO-RAs). In long-term studies on TPO-RAs, arterial and venous thrombosis occurred in 6% of treated patients. Rituximab, a monoclonal antibody directed against CD-20 on the B-cells is widely used as a treatment for ITP. It yields a response rate of 60% and is generally well-tolerated. However, to our knowledge VTE events following treatment with rituximab for ITP have not been reported.
Aims
We report thromboembolic adverse events encountered in 2 multicenter, randomized controlled trials (RCT) for adult ITP patients treated with rituximab: 1) Rituximab as second-line treatment for adult ITP (the RITP trial)(Ghanima et al, Lancet 2015; 385) and 2) The Rituximab Maintenance Therapy in ITP (The PROLONG Trial; NCT03010202)
Methods
RITP was a multicenter, randomized placebo-controlled trial in which adult patients with ITP unresponsive to corticosteroid and platelet count <30x10⁹/L were enrolled. Of the 112 ITP patients, 55 patients were randomly assigned to 4 weekly infusions of 375 mg/m2 rituximab and the remaining received placebo. Concurrent treatment with corticosteroids only was allowed during the study. The steroid dose had to be tapered to the lowest dose that kept the platelet count >20x10⁹/L.
PROLONG is an ongoing multicenter, two-phase RCT. In the first phase, patients with ITP, who fail to achieve an adequate response to corticosteroids (prednisolone or dexamethasone) during the first year after diagnosis are randomized to receive rituximab 1000 mg twice with 15 days apart with or without dexamethasone 20 mg for 4 days starting with rituximab. In the secondphase, responding patients will be randomized at 24 weeks to maintenance therapy with 2 rituximab or placebo infusions separated by 24 weeks.
Results
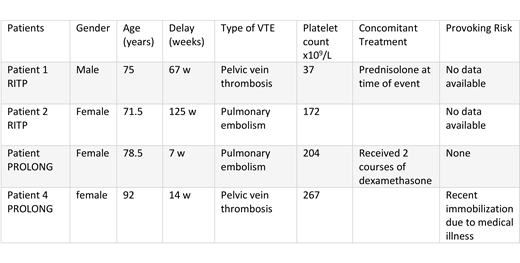
Four venous thromboembolic events were reported in 4 patients: 2 of 55 patients who received rituximab in the RITP trial and 2 (one received dexamethasone+rituximab) of 52 patients included so far in the first phase of the PROLONG trial, which results in a VTE rate of 3.7% in the rituximab treated patients. No thromboembolic events were encountered in the placebo arm of the RITP study (n=54 patients, matched on age). Median time from treatment to event was 40.5 weeks, with 2 events occurring 7-14 weeks after the administration of the first dose of rituximab. Median platelet count at the time of VTE was 188x109/L. Characteristics of the 4 patients are shown in the table; none was splenectomized or received thrombopoietic agents before the VTE event. No arterial thromboembolic events were reported in the 2 trials.
Conclusion
The rate of VTE following treatment with rituximab in these 2 studies seems to be higher than what is expected in an adult ITP population and the general population (Norgaard et al. Br J Haematol. 2016;174). However, based on these results we cannot determine whether the venous thromboembolic events were just incidental events or triggered by the treatment and/or the normalization of platelet count or the presence of risk factors for VTE as old age and immobilization.
Tvedt:Ablynx, alexion and novartis: Consultancy. Michel:Novartis: Consultancy; Amgen: Consultancy; Rigel: Consultancy. Ghanima:Bayer: Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Pfizer/BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal