
Introduction:
The gold standard for the diagnosis of absolute iron-deficiency anemia (IDA) in hemodialysis patients is a bone marrow aspirate with iron staining. Many clinicians use peripheral iron indices instead because they are non-invasive. Previous studies suggested that a serum ferritin < 200 ng/mL was a reliable indicator of absolute iron deficiency in the hemodialysis population. However, the sensitivity of serum ferritin for the diagnosis of IDA in hemodialysis patients is poor.
Methods:
The primary objective of this study was to identify the optimal ferritin value to diagnose patients with absolute iron deficiency, as assessed on bone marrow aspiration, in the renal dialysis population. Secondary endpoints included the rate of clinically relevant findings on gastrointestinal investigation according to iron status. Research Ethics Board approval was obtained from Queen's University for this retrospective chart review. Hematopathology laboratory records were used to determine all individuals who had bone marrow examination at Kingston Health Sciences tertiary referral center between 2008 January 1 and 2018 August 21. This list was cross-referenced with the Nephrology dialysis database to identify the pre-specified study cohort; those who were receiving concurrent hemodialysis or peritoneal dialysis. Iron deficiency was defined as reduced or absent iron stores on bone marrow aspirate with Perl's Prussian blue stain. Anemia was defined as hemoglobin <130 g/L in males and <120 g/L in females. Additional parameters collected included ferritin (normal range 22 - 275 ng/mL male and 4 - 205 ng/mL female), transferrin saturation (TSAT, normal range 20-55%), vitamin B12, folate, albumin, CRP and thyroid function tests. Peripheral iron indices over six months were analyzed; statistical analysis was performed with t-tests and Mann-Whitney U tests. ROC curves were generated to determine the sensitivity and specificity of various threshold values for serum ferritin and TSAT.
Results:
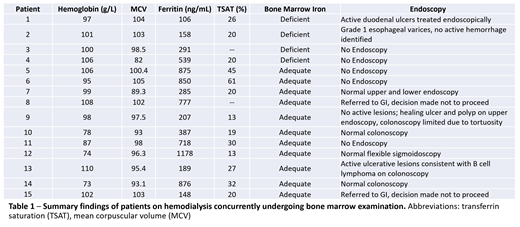
Between 2008 January 1 and 2018 August 21, 4234 patients underwent bone marrow examination, of whom 28 had received renal dialysis replacement therapy at some point. Fifteen patients concurrently at the time of bone marrow testing receiving hemodialysis form the study population (Table 1). Among these fifteen patients, 6 (40%) were female, median age was 70.5 (range 39 - 80) years and all were anemic (Hb range 73 - 110 g/L).
Four of these individuals were absolutely iron-deficient with reduced or absent iron stores by bone marrow evaluation. The mean ferritin and TSAT values for individuals with absolute iron deficiency by bone marrow aspiration was 273.5 ng/mL (n=4; median 224.5 ng/mL; range 158-539 ng/mL) and 22.0% (n=3; median 20%; range 20-26%), respectively. All four commenced erythropoietin stimulating agents; two received oral iron supplementation.
Eight patients, including two of those determined to be absolutely iron deficient on bone marrow, had endoscopic investigation. Two were identified to have sources of gastrointestinal bleeding, both with ferritin values in the 100-200 range (106 and 189 ng/mL).
With the limited sample size, the sensitivity and specificity of ferritin to identify absolute iron deficiency in this hemodialysis population was 50% and 85%, respectively, at a threshold of 198 ng/mL.
Discussion:
Ferritin and TSAT are not sensitive markers for absolute iron deficiency in hemodialysis patients. Bone marrow examination is performed in a minority. The small sample size in this study precludes definitive determination of an optimal ferritin cut-off to diagnose iron deficiency in the dialysis population. Uncertainty about actual iron status may result in alternative invasive testing, such as colonoscopy, to investigate the cause of their anemia.
Newer tests such as reticulocyte hemoglobin content and percent hypochromic red blood cells are more accurate and may guide diagnosis and management of IDA in hemodialysis patients. However they are not always routinely available. Further studies are needed to compare the utility of these peripheral iron indices to the gold standard bone marrow examination in a larger population, to allow identification of patients with absolute or functional IDA, and minimize invasive and potentially unnecessary investigation.
Hay:AbbVie: Research Funding; Kite: Research Funding; Janssen: Research Funding; Seattle Genetics: Research Funding; Celgene: Research Funding; MorphoSys: Research Funding; Roche: Research Funding; Novartis: Research Funding; Gilead: Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal