Introduction
Cold agglutinin disease (CAD) is a rare form of autoimmune hemolytic anemia (AIHA). It is characterized by both IgM-mediated agglutination of erythrocytes and hemolysis mediated by activation of the classical complement pathway. Recent studies have shown an increased risk of thromboembolic events (TE) in CAD patients. In addition, a recent analysis using the Danish National Patient Registry demonstrated a significant increase in mortality for CAD patients compared with matched individuals from the general population in Denmark (Bylsma et al, HemaSphere, 2018). Mortality among CAD patients has not been assessed in a US population. This study evaluated mortality risk in the largest cohort of CAD patients in the US.
Methods
Patients were retrospectively identified from the Optum® de-identified EHR dataset. Between January 2007 and September 2018 (study period), patients with ≥1 AIHA-related medical encounter and ≥3 mentions from physician notes of CAD-related terms ("cold agglutinin disease," "cold autoimmune hemolytic anemia," or "cold agglutinin hemoglobinuria") were included in the CAD cohort ("case"). For this cohort, the first mention of CAD terms was set as the index date. Patients without an AIHA-related medical encounter were included in the non-CAD cohort ("control"). For the control cohort, the index date was assigned based on the average occurrence of index date in the CAD population for the duration in the EHR database. For both cohorts, the baseline period was defined as the interval from the start of the medical activity in the EHR database or study period (whichever occurs later) to the index date, and the follow-up period was defined as the interval from the index date to the end of the study period, the end of medical activity, or death (whichever occurs earlier). The case and control cohorts were matched by age, gender, race, region, index year, and follow-up period using 1:5 nearest neighbor matching. Both cohorts were stratified according to the presence or absence of ≥1 TE during the study period. Mortality rate per 100,000 patients was calculated as the number of patients who died in each cohort divided by the number of patients in each cohort, from 2007 to 2018, multiplied by 100,000. Mortality rate was compared between matched cohorts using a Poisson test. An independent t-test was used to compare age at death between matched CAD and control groups; and time to death (starting from the index date) was analyzed using Kaplan-Meier curves and compared between matched cohorts using log-rank P test.
Results
In total, 651 CAD patients and 3,255 matched non-CAD controls were identified. Of these, 35% (n=228) of CAD patients and 20% (n=641) of non-CAD patients experienced ≥1 TE (P<0.001). Median age at index date for both cohorts was 72 years. Most patients were female (CAD 64%; non-CAD 65%) and Caucasian (CAD 85%; non-CAD 85%). Median follow-up duration was 42 months for the CAD cohort and 51 months for the control cohort. Mean (standard deviation [SD]) Elixhauser Comorbidity Index Score was 8.0 (4.9) for CAD patients and 4.5 (4.1) for matched controls.
The overall mortality rate was significantly higher for the CAD cohort than the matched-control cohort (CAD: 17,512 vs non-CAD: 11,306; P<0.001). For patients that experienced ≥1 TE during the study period, the mortality rate in the CAD cohort was 23,684 compared with 15,913 in the matched-control cohort (P<0.001; Table 1).
During the study period, 114 CAD patients and 368 matched non-CAD patients died. The mean (SD) age at death in the CAD cohort (77 [12] years) was lower compared with the matched controls (82 [8] years; P<0.001). For patients with ≥1 TE, mean (SD) age at death was 77 (13) years vs 82 (7) years for the CAD and control cohorts, respectively (P<0.001; Table 1).
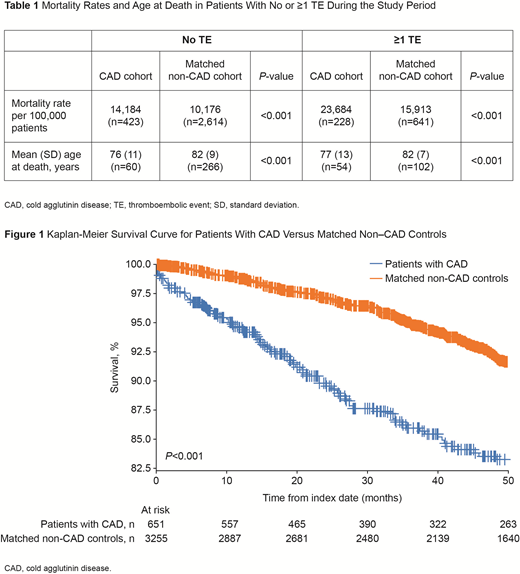
A Kaplan-Meier analysis demonstrated a significantly decreased survival probability among CAD patients compared with matched controls (P<0.001; Figure 1). In addition, CAD patients with ≥1 TE also had a significant decrease in survival when compared with matched controls with ≥1 TE (P<0.001).
Conclusions
CAD patients in the US have an increased mortality risk compared with a matched non-CAD population. The associated increased TE risk observed among CAD patients may be a contributing factor to this mortality. Further studies are needed to better define this association and elucidate other potential contributors to mortality in these patients.
Hill:Apellis: Honoraria; Novartis: Speakers Bureau; Bioverativ, a Sanofi company: Honoraria; Alexion: Research Funding. Punekar:Sanofi: Employment, Equity Ownership. Morales Arias:Sanofi: Employment, Equity Ownership. Broome:Cellphire: Research Funding; Alexion: Honoraria, Research Funding; Sanofi Genzyme: Honoraria, Research Funding; Incyte: Research Funding; Rigel: Research Funding. Su:Sanofi Genzyme: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal