Background:
Patients infected with HIV have an 8 to 150-fold risk of lymphoma compared to HIV uninfected patients, with certain aggressive non-Hodgkin lymphoma (NHL) histologies being AIDS-defining. Despite the decrease in incidence of both NHL and Hodgkin lymphoma (HL) in the current anti-retroviral treatment (ART) era, HIV-infected patients remain at significantly increased risk of lymphoma compared to the general population.
Over the last decade, advances have been made in the treatment of both NHL and HL with 18 agents approved since 2009. Unfortunately, safety and efficacy data in the HIV-infected population have been lacking in part due to exclusion of such patients from clinical trials. As such, the optimal treatment of HIV-associated lymphomas is unknown. In 2017, the National Comprehensive Care Network (NCCN) and the American Society of Clinical Oncology (ASCO) advocated expanding clinical trial opportunities for HIV-infected patients. However, exclusion criteria precluding HIV-infected patient participation likely persist. We sought to document and describe the current status of clinical trial exclusion criteria for NHL and HL as they relate to HIV infection.
Methods
We extracted data from the National Institute of Health's (NIH) registry of clinical trials (ClinicalTrials.gov) using the search term 'lymphoma' and reviewed all clinical trials (independent of funding source) in the United States that were recruiting or not yet recruiting adult patients for therapeutic trials (phase 1, 2, or 3) as of June 2019. A total of 734 trials met the above criteria; of these 206 were excluded as they were not primarily adult lymphoma trials (allogeneic transplant [n=87], leukemia [n=79], pediatric [n=87], lung cancer [n=12], and other [n=12]). As such, 526 clinical trials were included in the final sample.
The primary outcome measure was whether HIV infection was an exclusion criterion for participation in the clinical trial. With the exception of the sample size, which was measured as a continuous variable, explanatory variables were dichotomized and included funding source (NIH vs. non-NIH), phase (phase 1 vs. phase 2 or 3), start date (start date prior to January 2018 vs. start date on or after January 2018), and recruitment status (recruiting vs. not yet recruiting). If the lymphoma under study was an aggressive subtype it was dichotomized as an AIDS-defining malignancy (aggressive NHL vs. other).
We assessed whether these explanatory variables were associated with the primary outcome measure. We utilized bivariate analyses to identify measures with p < 0.20 for inclusion in a multivariate logistic regression model.
Results:
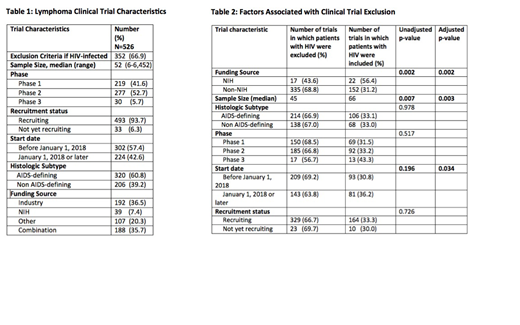
Of the 526 lymphoma-related trials, 352 (66.9%) excluded HIV-infected patients. Among all studies, the target sample sizes of these studies ranged from 6 to 6,542 (median 52) and were primarily phase 1 (219; 41.6%) or phase 2 (277; 52.7%) studies. Only 30 of the clinical trials were phase 3 (5.7%). The majority of studies (493; 93.7%) were actively recruiting and were initiated prior to January 1, 2018 (302; 57.4%). The majority of the 526 trials (320; 60.8%) included patients with aggressive NHL. The remaining trials included patients with indolent lymphoma, HL, or NHL with an unspecified subtype. Only 39 (7.4%) of all studies were funded strictly by NIH; the remainder were funded strictly by industry (192; 36.5%), 'other' (107; 20.3%), or a combination (188; 35.7%).[Table 1]
Of the explanatory variables, funding source, sample size, and a start date on or after January 1, 2018 were associated with the outcome measure in the bivariate analysis and were included in a multivariate model. In the multivariate analyses, NIH-funded studies (OR 0.34 [0.17, 0.67], studies which began recruitment after January 1, 2018 (OR 0.66 [0.45, 0.97]) and larger studies (OR 0.998 [0.996, 0.999] were less likely to exclude HIV-infected patients. [Table 2]
Conclusions:
Despite support from ASCO and specific NCCN guidance which advocates that the participation in cancer clinical trials of patients living with HIV/AIDS "should be encouraged whenever feasible," the majority of interventional clinical trials for the treatment of lymphoma currently exclude participants based on infection with HIV. This goal of inclusion could be accomplished by adding an additional HIV-specific safety and efficacy cohort to most studies which could potentially be mandated by the FDA for all registrational trials.
Lynch:Rhizen Pharmaceuticals S.A: Research Funding; Takeda Pharmaceuticals: Research Funding; T.G. Therapeutics: Research Funding; Incyte Corporation: Research Funding; Johnson Graffe Keay Moniz & Wick LLP: Consultancy; Juno Therapeutics: Research Funding. Shadman:Sound Biologics: Consultancy; Acerta: Research Funding; Pharmacyclics: Consultancy, Research Funding; Celgene: Research Funding; Gilead: Research Funding; TG Therapeutics: Research Funding; Atara: Consultancy; Verastem: Consultancy; Mustang Biopharma: Research Funding; Bigene: Research Funding; ADC Therapeutics: Consultancy; Merck: Research Funding; AbbVIe: Consultancy, Research Funding; Sunesis: Research Funding; Genentech, Inc.: Consultancy, Research Funding; Emergent: Research Funding; AstraZeneca: Consultancy. Shustov:Spectrum Pharmaceuticals: Consultancy, Research Funding. Smith:AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma BV: Research Funding; Merck Sharp & Dohme Corp: Consultancy, Research Funding; Denovo Biopharma: Research Funding; Genentech: Research Funding; Ignyta (spouse): Research Funding; Bristol-Myers Squibb (spouse): Research Funding; Ayala (spouse): Research Funding; Pharmacyclics: Research Funding; Portola Pharmaceuticals: Research Funding; Seattle Genetics: Research Funding; Incyte Corporation: Research Funding. Till:Mustang Bio: Patents & Royalties, Research Funding. Ujjani:PCYC: Research Funding; Pharmacyclics: Honoraria; Pharmacyclics: Honoraria; Genentech: Honoraria; PCYC: Research Funding; Gilead: Consultancy; Gilead: Consultancy; Astrazeneca: Consultancy; Astrazeneca: Consultancy; Atara: Consultancy; Genentech: Honoraria; Atara: Consultancy; AbbVie: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding. Uldrick:Roche: Other: commercial research support through a CTA with Fred Hutchinson Cancer Research Center; Merck: Other: drug for a clinical trial from Merck through a CRADA with the NCI; Celgene: Other: research support from Celgene through a CRADA at the NCI; Patent: Patents & Royalties: co-inventor on US Patent 10,001,483 entitled . Gopal:Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte.: Consultancy; Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte: Honoraria; Teva, Bristol-Myers Squibb, Merck, Takeda, Seattle Genetics, Pfizer, Janssen, Takeda, and Effector: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal