Background: Patients with classical Hodgkin lymphoma (cHL) relapsed or refractory (R/R) disease who relapse after or are ineligible for autologous stem cell transplantation have a poor prognosis. Recently, the anti-PD1 monoclonal antibodies nivolumab and pembrolizumab were approved by the FDA (May 2016 and March 2017, respectively) as treatment options for R/R cHL patients. In the absence of head-to-head clinical trials, observational data may provide hypothesis-generating insight into the real-world outcomes of patients receiving these PD-1 inhibitors. This study aims to evaluate healthcare resource utilization (HRU) among patients with cHL initiated on pembrolizumab compared to nivolumab in the United States.
Methods: A retrospective database analysis was conducted using Symphony Health's Patient Integrated Dataverse® (07/2014-06/2018). The date of the first dispensing or administration of pembrolizumab or nivolumab was assigned as the index date. Patients who received both treatments and who initiated nivolumab prior to pembrolizumab approval, or in the first months after, were classified in the pembrolizumab cohort. Included patients were required to meet the following criteria: ≥12 months of continuous clinical activity prior to the index date, ≥1 inpatient or ≥2 outpatient visits with a cHL diagnosis prior to the index date, no diagnosis of nodular lymphocyte-predominant Hodgkin lymphoma, and ≥18 years of age at the index date. Baseline patient characteristics were assessed in the 12-month baseline prior to the index date. Inverse probability of treatment weighting (IPTW) based on the propensity score was used to adjust for observed differences in baseline covariates between cohorts. Rates per person-year (PPY) of all-cause and cHL-related (i.e., visits with a primary or secondary diagnosis of cHL) HRU were calculated for weighted cohorts during the follow-up period, which spanned from the index date to the end of clinical activity or data availability. Rates of HRU were compared using rate ratios (RRs) from Poisson regression models.
Results: A total of 92 patients initiated on pembrolizumab and 218 patients initiated on nivolumab met the selection criteria and were included in the analysis. Of the 92 pembrolizumab patients, 6 patients received nivolumab during the baseline period. After weighting, the mean age was similar at 55 years in both cohorts, while the proportion of female was lower in the pembrolizumab cohort (35.3%) compared to the nivolumab cohort (44.1%; standardized difference [StD]=17.9%). The mean (median) follow-up period was 295 (264) days for pembrolizumab users and 274 (208) days for nivolumab users (StD=9.4%). Mean Quan-Charlson Comorbidity Index score was well balanced after weighting in the pembrolizumab and nivolumab cohorts (4.2 and 4.3, respectively; StD=2.2%); 13.8% and 15.0% had depressive disorders (StD=3.7%), and 8.5% and 10.2% had substance-related and addictive disorders (StD=5.8%), respectively. The mean number of baseline hospitalizations (pembrolizumab=1.6, nivolumab=1.5; StD=3.1%) was also well balanced after weighting.
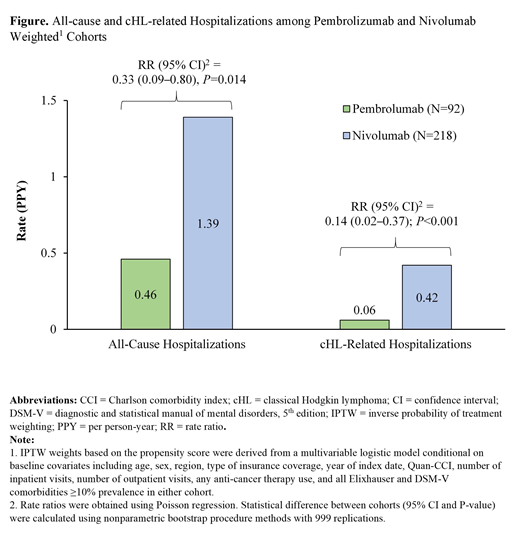
The total person-time of observation was 74.6 and 163.6 years for the weighted pembrolizumab and nivolumab cohorts, respectively. Over this period, patients in the pembrolizumab cohort had significantly lower rates of all-cause hospitalizations (0.46) PPY than those in the nivolumab cohort (1.39; RR [95% confidence interval, CI]=0.33 [0.09-0.80], P=0.014; Figure). Furthermore, the rate of cHL-related hospitalizations PPY was significantly lower in the pembrolizumab cohort (0.06) compared to the nivolumab cohort (0.42; RR [95% CI]=0.14 [0.02-0.37], P<0.001; Figure). A similar trend (i.e., ratio below 1) was observed for all-cause and cHL-related outpatient visits, but the difference was not statistically significant (all-cause RR [95% CI]=0.84 [0.56-1.11], P=0.200; cHL-related RR [95% CI]=0.90 [0.47-1.42], P=0.647).
Conclusion: In this real-world study, adult cHL patients treated with pembrolizumab had significantly lower rates of all-cause and cHL-related hospitalizations than those treated with nivolumab. Fewer all-cause and cHL-related outpatient visits were also observed among pembrolizumab users. Additional research is warranted to further investigate these early trends in real-world HRU observed among patients with cHL receiving anti-PD1 therapies.
Laliberté:Janssen Scientific Affairs, LLC: Research Funding; Merck & Co., Inc.: Research Funding. Raut:Merck & Co., Inc.: Employment. Yang:Merck & Co.: Employment. Germain:Janssen Scientific Affairs, LLC: Research Funding; Merck & Co., Inc.: Research Funding. Sen:Merck & Co., Inc.: Employment. MacKnight:Merck & Co., Inc.: Research Funding; Janssen Scientific Affairs, LLC: Research Funding. Desai:Merck & Co., Inc.: Employment. Duh:Analysis Group, Inc., a consulting firm that has received research funding from Shire, a Takeda company, to conduct this study: Employment; Shire: Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal