Introduction: Randomized controlled trials (RCTs) have shown similar yet conflicting primary outcomes for the use of low-dose direct oral anticoagulant (DOAC), including rivaroxaban and apixaban, in the prevention of cancer-associated thrombosis (CAT). It remains unclear if this preventive strategy is consistent, robust, and cost-effective across different trial populations.
Methods: We performed a systematic review of RCTs that compared DOAC vs. placebo for CAT prevention using EMBASE, MEDLINE, and CENTRAL. Two authors screened/reviewed articles and abstracted the data. Primary efficacy, sensitivity efficacy, and safety outcomes were uniformly defined and extracted from the studies. Meta-analysis was performed using random-effects model. Subgroup analysis was performed for patients with intermediate- and high-risk Khorana Score. Using inputs from the meta-analysis and relevant epidemiology and outcomes studies, we performed a cost-utility analysis using a Markov state-transition model over life time in a hypothetical cohort of 60-year-old high-risk patients with a similar distribution of cancers as the pooled RCTs. We calculated the differences in cost, quality-adjusted life year (QALY), and incremental cost-effectiveness ratio (ICER) and performed one-way and probabilistic sensitivity analyses to test the robustness of the results.
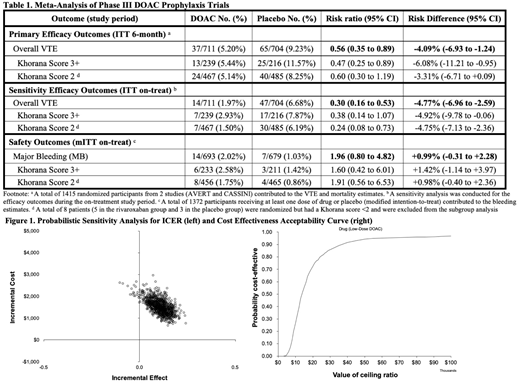
Results: A total of 202 records were identified and 28 full-text articles were assessed. Two studies with 1415 participants were eligible to be included for meta-analysis (Table 1). For DOAC vs. placebo, the relative risk (RR) for overall VTE incidence by six months was 0.56 (0.35-0.89). The RR for major bleeding (MB) on-treatment was 1.96 (0.80-4.82). Patients with high-risk Khorana score (3+) derived the largest absolute risk reduction of VTE. Based on a Markov model, in patients at intermediate-to-high risk for VTE (Khorana Score 2+), prophylaxis with low-dose DOAC thromboprophylaxis for 6 months, compared to placebo, was associated with 32 per 1000 fewer CAT events and 11 per 1000 more MB over life-time. Prophylaxis was associated with an incremental cost increase of $1,445 and an incremental QALY increase of 0.12, resulting in an ICER of $11,947 per QALY gained (Figure 1). Key drivers of ICER variation included the relative risks of VTE and major bleeding as well as the cost of drug. Based on the cost-effectiveness acceptability curve, this preventive strategy was 94% cost effective at the threshold of $50,000. In a scenario sensitivity analysis, patients with the highest risk of VTE (Khorana Score 3+) derived the most benefit from low-dose DOAC thromboprophylaxis.
Conclusion: Low-dose DOAC (rivaroxaban or apixaban) thromboprophylaxis for 6 months reduces the rate of overall VTE in higher-risk cancer patients starting systemic chemotherapy and may increase the likelihood of bleeding. It appears to be a cost-effective strategy for the prevention of CAT in intermediate-to-high risk ambulatory patients based on our analyses; however, the differential impact on QALY is small over lifetime. Future research should focus on a better understanding of the significance of these adverse events on longer term quality of life and their impact on delays in anti-cancer treatment.
Kuderer:Celldex: Consultancy; Halozyme: Consultancy; Coherus Biosciences: Consultancy, Other: Travel, Accommodations, Expenses; Mylan: Consultancy, Other: Travel, Accommodations, Expenses; Pfizer: Consultancy; Myriad Genetics: Consultancy; Janssen Scientific Affairs, LLC: Consultancy, Other: Travel, Accommodations, Expenses. Khorana:Janssen: Consultancy; Bayer: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy. Carrier:Leo Pharma: Honoraria, Research Funding; Servier: Honoraria; Bayer: Honoraria; Pfizer: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Lyman:G1 Therapeutics, Halozyme Therapeutics, Partners Healthcare, Hexal, Bristol-Myers Squibb, Helsinn Therapeutics, Amgen Inc., Pfizer, Agendia, Genomic Health, Inc.: Consultancy; Amgen Inc.: Other: Research support, Research Funding; Generex Biotechnology: Membership on an entity's Board of Directors or advisory committees; Janssen Scientific Affairs, LLC: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal