Introduction: Immune thrombocytopenia (ITP) is an autoimmune disorder that is characterized by low platelet count, and that often requires rescue therapy (e.g., corticosteroids, intravenous immunoglobulin [IVIG]) and can lead to serious bleeding-related episodes (BREs). Based on the International Working Group (IWG), ITP is classified into three groups by duration of disease: newly diagnosed (0 to <3 months), persistent (≥3 to ≤12 months), and chronic (>12 months); patients in the newly diagnosed or persistent phase are considered to have early ITP. Romiplostim, an injectable thrombopoietin receptor agonist, has demonstrated efficacy in clinical trials of adults with chronic ITP. Evidence of drug efficacy from clinical trials of adults with chronic ITP may not, however, be reflective of drug effectiveness among adults with early ITP in clinical practice. The objective of this study was to evaluate the effectiveness of romiplostim for the treatment of adults with early ITP in real-world settings.
Methods: A retrospective pre-post cohort design and data from a large healthcare claims repository (2009-2017) were employed. The study population comprised patients aged ≥18 years who initiated romiplostim and, during the 1-year period prior to therapy initiation ("pre-period"), had their first diagnosis of ITP and evidence of a BRE. Patients were excluded from the study population if they were not continuously enrolled during the 1-year pre-period and 1-year period following therapy initiation ("post-period") or if, during the pre-period, they had evidence of conditions (e.g., viral hepatitis B, hepatitis C, HIV, lupus, pancytopenia, cancer) that may lead to secondary thrombocytopenia. Study outcomes included BREs, use of rescue medication (oral/IV corticosteroids, IVIG, anti-RhD immunoglobulin [anti-D], platelet transfusions), splenectomy, and thrombocytopenia-related hospitalizations, and were compared between the 1-year maximum pre-period (depending on timing of initial ITP evidence relative to initiation of romiplostim) and the 1-year post-period using the McNemar test and paired-samples t-test.
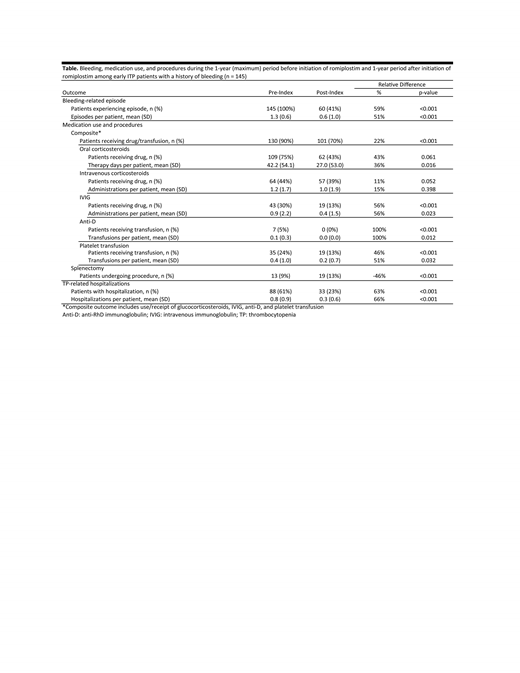
Results: The study population included 145 adults with early ITP who initiated romiplostim and had a history of BREs; mean age was 54 years, 54% were female, and 57% were in the newly diagnosed phase at the time of therapy initiation (duration from first ITP evidence to initiation of romiplostim: mean = 166 days, range = 4 - 365 days). Common acute and chronic medical conditions among patients in the study population included cardiovascular disease (24%), diabetes (21%), anemia (30%), and osteoarthritis (11%). From the variable pre-period to the 1-year post-period, incidence of BREs requiring a healthcare encounter decreased by 59% (p<0.001) and use of rescue therapy decreased by 22% (p<0.001) on an overall basis, ranging from 11% (p=0.052) for IV glucocorticosteroids to 100% (p<0.001) for anti-D (Table). Thrombocytopenia-related hospitalizations decreased by 63% (p<0.001); splenectomy increased from 9% to 13% (p<0.001).
Conclusions: In this real-world evaluation of adults with early ITP and a bleeding history who initiated romiplostim in US clinical practice, marked decreases were observed in the incidence of BREs, use of rescue therapy, and thrombocytopenia-related hospitalizations following initiation of romiplostim.
Hatfield:Amgen Inc.: Employment, Equity Ownership. Shah:Amgen Inc.: Employment, Equity Ownership. Lawrence:Amgen Inc.: Employment, Equity Ownership. Hanau:Amgen Inc.: Research Funding. Lonshteyn:Amgen Inc.: Research Funding. Weycker:Amgen Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal