Background
Patients with transfusion-dependent β-thalassemia (TDT) may experience transfusional iron overload and end-organ damage. While potentially curative, allogeneic hematopoietic stem cell (HSC) transplantation is limited by transplant-related risks and donor availability. Transplantation of autologous CD34+ cells encoding a βA-T87Q-globin gene (LentiGlobin gene therapy for β-thalassemia) may overcome some of these limitations. βA-T87Q-globin is incorporated into adult hemoglobin (Hb), forming gene therapy-derived HbAT87Q, which can be distinguished from other Hb species. The phase 1/2 Northstar study (HGB-204; NCT01745120) using the original manufacturing process evaluated the safety and efficacy of LentiGlobin in adolescents and adults with TDT (≥100 mL/kg/yr of red blood cells [RBCs] or ≥8 RBC transfusions/yr) and non-β0/β0 or β0/β0 genotypes.
Methods
HSCs were mobilized with G-CSF and plerixafor and collected via apheresis. CD34+ cells were transduced with BB305 lentiviral vector. After busulfan myeloablation, patients were infused with transduced cells. Primary efficacy endpoints were sustained production of ≥2 g/dL HbAT87Q between months 18 and 24 and transfusion independence (TI; weighted average Hb ≥9 g/dL without RBC transfusions for ≥12 months). Patients were monitored for 2 years and subsequently enrolled in the 13-year long-term follow-up study, LTF-303 (NCT02633943). Results are shown as median (min ‒ max) unless otherwise indicated.
Results
Eighteen patients were treated (age: 20 [12 - 35] yrs) and followed for 40.7 (29.3 - 53.8) months as of 13 December 2018. In the 2 years prior to enrollment, patients had an annualized transfusion volume of 169.0 (124.0 - 273.0) mL/kg/yr and pre-transfusion weighted mean nadir Hb of 9.3 (7.0 - 10.1) g/dL.
Neutrophil and platelet engraftment occurred at 18.5 (14 - 30) and 39.5 (19 - 191) days, respectively. No patient had graft failure. Grade ≥3 non-hematologic adverse events (AEs) reported in ≥25% of patients after infusion were stomatitis, febrile neutropenia, and pharyngeal inflammation. No replication-competent lentivirus or death has been reported. The vector integration site profile in all 18 patients has remained polyclonal. The number of unique integration sites (UIS) identified was 1646 (190 - 2888), 1677 (151 - 6935), 2484 (984 - 5511), 1773 (1260 - 2693) at Months 12 (n=18), 24 (n=18), 36 (n=11), 48 (n=4), respectively. The highest mean (SD) frequency of any UIS in patients across all visits was 11.5% (5.8%). No oncogenesis has been reported.
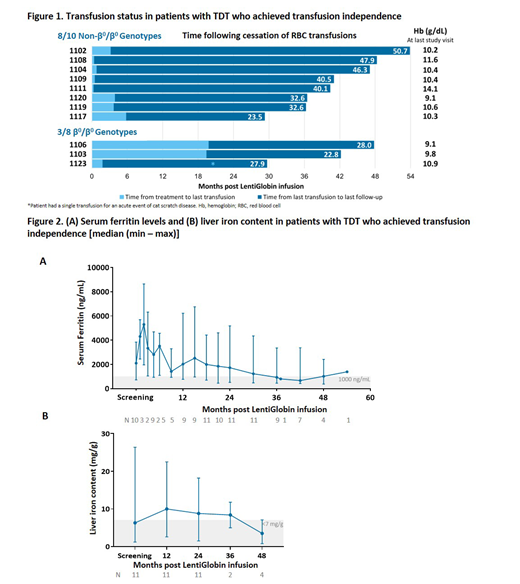
In Northstar, 16/18 (89%) patients achieved the primary endpoint of ≥2 g/dL HbAT87Q between months 18 and 24. Eight of 10 (80%) patients with non-β0/β0 genotypes achieved and maintained TI; current duration of TI was 38 (21.2 - 45.3) months (Figure 1). The weighted average total Hb during TI was 10.3 (9.1 - 13.2) g/dL. Total Hb and HbAT87Q remained stable over time. Total Hb in patients with non-β0/β0 genotypes who achieved TI was 10.3, 10.4, 10.6, and 11.1 g/dL at Months 12 (n=8), 24 (n=8), 36 (n=7), 48 (n=3), respectively. Transfusion volumes were reduced by 73% and 43% in the 2 patients still receiving transfusions.
Three of 8 (38%) patients with β0/β0 genotypes achieved TI with a current duration of 16.4 (16.1 - 20.8) months. Weighted average total Hb during TI was 9.9 (9.5 - 10.1) g/dL and HbAT87Q was 8.0 - 8.9 g/dL at last visit. One additional patient was transfusion-free for 13.7 months; however, total Hb was <9 g/dL. The 4 other patients had a transfusion volume reduction of 53% (10% - 72%).
Patients who achieved TI resumed iron chelation 13 (2 - 15) months after infusion and all remain on iron chelation as of last follow-up. Serum ferritin and liver iron content (LIC) (Figure 2A, 2B) were reduced in patients who achieved TI by 55% (16 - 78%) and 56% (38 - 83%) from screening to Month 48 (n=4), respectively. Of these 4 patients who had a Month 48 visit, LIC values were 0.8 - 7.1 mg/g at Month 48 compared to 4.8 - 11.5 mg/g at screening. In patients who achieved TI, cardiac T2* ranged from 27.0 - 39.0 msec at screening and 31.4 - 57.6 msec at last visit.
Summary
With up to 4.5 years of follow-up after LentiGlobin gene therapy, generally stable HbAT87Q levels and durable TI were observed in 8/10 and 3/8 patients with TDT and non-β0/β0 and β0/β0 genotypes, respectively. Iron burden has improved over time in patients who achieved TI. The safety profile of LentiGlobin remains consistent with myeloablative conditioning.
Kwiatkowski:Imara: Consultancy; Agios: Consultancy; bluebird bio, Inc.: Consultancy, Research Funding; Terumo: Research Funding; Apopharma: Research Funding; Novartis: Research Funding; Celgene: Consultancy. Thompson:bluebird bio, Inc.: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Baxalta: Research Funding. Rasko:GSK: Honoraria; bluebird bio: Honoraria; Imago: Consultancy; Novartis: Honoraria; Cynata: Honoraria; Spark: Honoraria; Takeda: Honoraria; NHMRC Mitochondrial Donation Expert Working Committee: Other: Advisory Committee; Gilead: Honoraria; Cure The Future Foundation: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Genea: Equity Ownership; Rarecyte: Consultancy, Equity Ownership; Gene Technology Technical Advisory, Australian Government: Other: Advisory committee; Celgene: Honoraria; Advisory Committee on Biologics, Australian Government: Other: Advisory Committee; Australian Cancer Research Scientific Advisory Board: Membership on an entity's Board of Directors or advisory committees; FSHD Global Research Foundation: Membership on an entity's Board of Directors or advisory committees. Schiller:Amgen: Other, Research Funding; Agios: Research Funding, Speakers Bureau; Astellas: Research Funding; Biomed Valley Discoveries: Research Funding; Bristol Myer Squibb: Research Funding; Celgene: Research Funding, Speakers Bureau; Constellation Pharmaceutical: Research Funding; Daiichi Sankyo: Research Funding; Eli Lilly and Company: Research Funding; FujiFilm: Research Funding; Genzyme: Research Funding; Gilead: Research Funding; Incyte: Research Funding; J&J: Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Onconova: Research Funding; Pfizer Pharmaceuticals: Equity Ownership, Research Funding; Sangamo Therapeutics: Research Funding. Cavazzana:Smartimmune: Other: Founder of Smartimmune. Ho:Celgene: Other: investigator meeting travel costs; Janssen: Other: investigator meeting travel costs; Novartis: Other: investigator meeting travel costs; La Jolla: Other: investigator meeting travel costs. Schmidt:German Cancer Research Center, Heidelberg, Germany: Employment; GeneWerk GmbH, Heidelberg, Gemrany: Equity Ownership. Vichinsky:Agios: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; GBT: Consultancy, Research Funding; bluebird bio: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Deary:bluebird bio, Inc.: Employment, Equity Ownership. Chen:bluebird bio, Inc.: Consultancy. Petrusich:bluebird bio, Inc.: Employment, Equity Ownership. Walters:Editas Medicine: Consultancy; TruCode: Consultancy; AllCells, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal