Background: Unrelated cord blood (UCB) transplant (UCBT) is not recommended in patients with thalassemia major (TM) so far. Post-transplant (PT) Cyclophosphamide (PTCy) with long pre-transplant immunosuppression therapy have improved haploidentical peripheral blood (PB) stem cell transplant (haplo-SCT) survival in TM patients but with 2/31 primary rejection. So, we designed a novel dual transplantation of UCBT following haplo-SCTwith PTCy(NF-14-TM-CT protocol).
Aim:To improve results of haplo-SCT and UCBT in patients with TM.
Patients and method: NF-14-TM -CT protocol was termed as double-insurance dual transplantsincluding a haplo-SCT and an UCBT, in which conditioning regimen consisted of ATG (at -10 to -8 day), Cy (-7), Fludarabine (-6 to -2), Busulfan (-6 to -4) Thiotepa (-3), haplo-PB (0), PTCy (+3, +4) and UCB (+6). PTCy serve as GVHD prophylaxes after haplo-SCT and as conditioning before UCBT. In total 131 patients with TM from three pediatric center in China received NF-14-TM-CT protocolfrom June, 2014 to April, 2019, with a median follow-up of 13 (2-59) months and a median age of 8 (3.5-17) years.
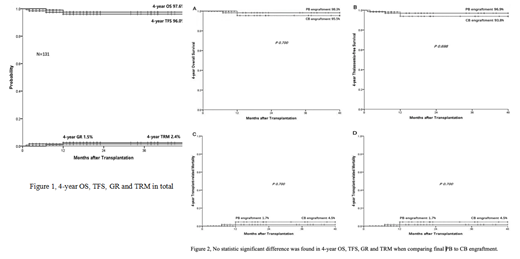
Results Final haplo-PB engrafted(group1)in 76 patients with mean PBSC-MNCof 22.49 (±5.36) x108/kgand UCB nuclear cells (NC) of 5.95 (±3.39) x107/kg and final UCB engrafted (group 2) in 55 patients with mean PBSC-MNC of 21.78 (±5.68) x108/kg and mean UCB-NC of. 5.43 (±2.32) x107/kg. The 4-year overall survival (OS), thalassemia-free survival (TFS), graft rejection (GR), and transplant related mortality (TRM) were 97.6%, 96.0%, 1.5%, and 2.4%, respectively (Fig. A), in total. The corresponding rates for group 1 were 98.3%, 96.9%, 1.7% and 1.8% and for group 2 were 95.5%, 93.8%, 4.5% and 1.4%, respectively. No statistic significant difference was found in OS, TFS, GR and TRM, respectively, when comparing group 1 with group 2 (Fig. B. C, D, E).The incidence of grade II-IV acute GVHD, III-IV acute GVHD, mild chronic GVHD, moderate/severe chronic GVHD, VOD, PT cystitisand PT hemolysis of the entire cohort was 16.8%, 6.87%, 9.92%, 1.52%, 4.60%, 31.3% and 14.5, respectively.
Summary:Current study proved that the novel CT improved the results of haplo-SCT and UCBT in patients with TM.
Wing:Miltenyi Biotec: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal