Background: Light chain (AL) amyloidosis is characterized by deposition of misfolded monoclonal immunoglobulin light chains leading to organ dysfunction. AL amyloidosis has traditionally been treated with agents used in multiple myeloma, primarily alkylators, proteasome inhibitors (PIs), immunomodulatory agents (IMiDs), and high-dose melphalan/autologous stem cell transplantation (ASCT). A retrospective study comparing patients with AL amyloidosis who underwent ASCT as frontline therapy ('upfront ASCT') to those undergoing ASCT following induction ('deferred ASCT') revealed that deferred ASCT was associated with prolonged overall survival (OS) compared to upfront ASCT (Afrough et al, Biol Blood Marrow Transplant, 2018). Given the number of effective new therapies for AL amyloidosis, the potential to delay ASCT after one or more lines of therapy ('salvage ASCT') is feasible. To our knowledge, transplant outcomes of AL amyloidosis patients undergoing deferred vs salvage ASCT following at least one relapse have not been reported. A retrospective chart review was conducted to compare AL amyloidosis patients receiving deferred vs salvage ASCT for progression-free survival (PFS) and overall survival (OS). The study was approved by the Institutional Review Board at Weill Cornell Medical College.
Methods: Twenty-four patients with AL amyloidosis who underwent deferred or salvage ASCT between 2000-2018 were included in the analysis. Patients who underwent upfront ASCT without induction chemotherapy were excluded. Demographics and clinical parameters were extracted from the electronic medical record. PFS was calculated from date of ASCT to first relapse and OS was calculated both from date of diagnosis and date of ASCT to death. Patients were censored if lost to follow-up prior to experiencing the relevant event. PFS as well as OS of patients who underwent deferred vs salvage ASCT were compared. Log-rank tests were used to statistically evaluate differences between Kaplan-Meier PFS/OS curves. Cox proportional hazards models were used to calculate hazard ratios (HR) using deferred ASCT as the reference treatment.
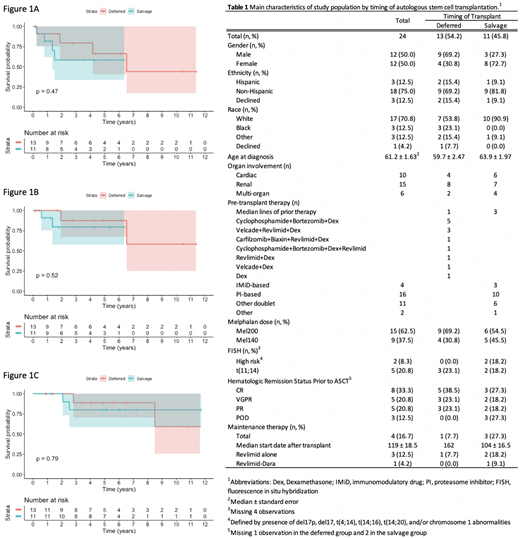
Results: Among the 24 patients included in this analysis, 13 underwent deferred ASCT with 1 prior line of chemotherapy (e.g., induction), and 11 underwent salvage ASCT with a median of 3 prior lines (Table 1). Ten patients had cardiac amyloidosis, 15 had renal amyloidosis, and 6 had multi-organ involvement. Induction regimens received in the deferred group are included in Table 1. Neither PFS nor OS was significantly different between patients receiving deferred vs salvage ASCT. After median follow-up of 2.9 years, median PFS in patients who received deferred ASCT was 6.5 years and not reached in those who received salvage ASCT (P=0.47), with a HR of 1.74 (95% CI, 0.38-7.85) (Figure 1A). Median OS from date of ASCT was not reached in either group after a median follow-up of 2.8 years (P=0.52), with a HR of 2.16 (95% CI, 0.19-24.04) (Figure 1B). Similarly, median OS calculated from date of diagnosis was not significantly different between deferred vs salvage ASCT (P=0.79) (Figure 1C).
Conclusions: In this cohort of 24 AL amyloidosis patients, no significant differences in PFS or OS were seen between patients undergoing deferred ASCT following induction vs salvage ASCT following multiple lines of therapy. Unlike the superior OS seen with deferred vs upfront ASCT, our findings show that either deferred or salvage ASCT may be associated with comparable outcomes and suggest similar OS despite timing of transplant. However, it is important to note the small sample size and that none of our patients received daratumumab-based regimens which may have improved PFS/OS in either or both groups. There were also small differences between groups in use of maintenance (1 vs 3 patients in the deferred and salvage groups, respectively) and proportion of patients receiving higher doses of melphalan 200mg/m2 vs 140 mg/m2 (69% vs 55% in deferred and salvage groups, respectively). Nevertheless, the lack of difference seen in PFS and OS between the two groups suggests that eventual hematologic relapse and organ progression with death occur at similar time intervals despite timing of ASCT. This speaks to the point that in newly diagnosed AL amyloidosis as well as later in the disease, achieving deep responses to prevent organ progression and death regardless of timing of ASCT remains an important treatment goal.
Van Besien:Miltenyi Biotec: Research Funding. Coleman:Kite Pharmaceuticals: Equity Ownership; Pharmacyclics: Speakers Bureau; Merck: Research Funding; Gilead, Bayer, Celgene: Consultancy, Research Funding, Speakers Bureau. Niesvizky:Takeda, Amgen, BMS, Janssen, Celgene: Consultancy, Research Funding. Rosenbaum:Janssen: Research Funding; Honoraria Akcea: Other: Accordant Health.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal