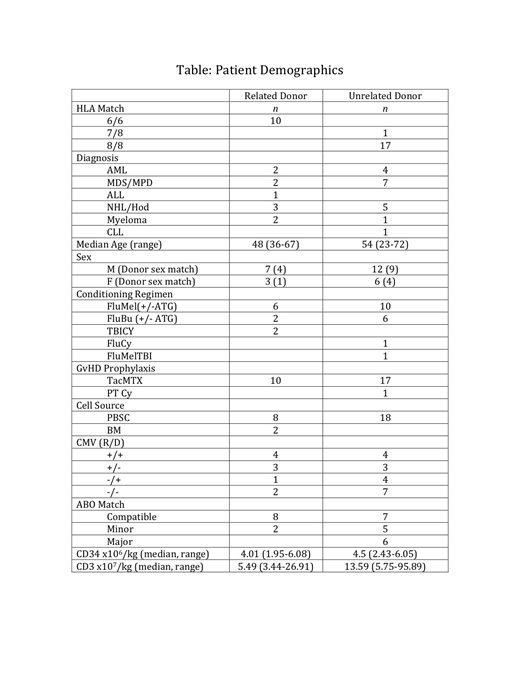
Telmisartan (TMS) is an angiotensin receptor blocker with anti-inflammatory properties and little or no clinical immunosuppressive effect. We undertook an unblinded, single arm, pilot study of TMS (160 mg/d p.o.) for prevention of acute graft vs. host disease (aGvHD) in recipients of HLA matched allogeneic hematopoietic stem cell transplants (HSCTs). Primary endpoints of Grade II or >Grade III aGvHD (1994 Consensus Conference on Acute GVHD Grading) were compared with 178 similar HSCT historical controls over the preceding 3 yrs at our institution: 26% >Grade III, 74% Grade II. Safety endpoints included engraftment, relapse/progression by day (d) 180, non-relapse mortality by d 180, and serious drug-related adverse reactions. Experimental data were collected on blood endotoxin levels and stool microbiome composition. The study was powered to detect 20% or 50% reduced rates of, respectively, Grade II or >Grade III. We report results from a planned interim analysis triggered when the first 22 evaluable patients (pts) reached d 100. Of 27 evaluable pts, 24 were beyond d 180 by completion of this interim analysis.
The study was designed to treat pts for two days prior to, and day of HSCT, and for an additional 98 days post-transplant, but pts were considered evaluable if they completed >14 days of post- transplant TMS, including pre-determined dose reductions. One enrollee received TMS for 13 days post-HSCT and was excluded from analysis. Five pts received 14-24 days of drug (4 by choice, 1 died of sepsis). More than half the evaluable patients (17/27) completed >94 days of post-HSCT therapy, including 6 who experienced an episode of hypotension. Almost half (13/27) received >80% of the full 101 day dose (16,160 mg), including 5 who experienced hypotension. Of the 6 enrollees receiving <24 days of drug, two had documented hypotension. No other serious drug-attributed adverse reactions were reported. Reasons cited for pts choosing to discontinue TMS were inconvenience of taking pills, and home stool sampling.
Safety Endpoints: All pts engrafted promptly (ANC >500/uL, platelet >20,000/uL). Peripheral donor CD3+ T cell median and range of chimerism on days 28 and 84 were 99.5% (37-100) and 100.0% (36-100); bone marrow donor CD34+ cell chimerism was 97.0% (62-100). There was a single NRM during the 180 d study, and no graft rejection or increase in relapse or progression. Four relapses occurred within 180 days, and 2 more after d 180, resulting in two relapse deaths (d 370, and d 600).
Primary Endpoints:A single individual developed hyperacute Grade III skin GvHD on day 7 p-HSCT. Another 12 of 27 patients developed Grade 2 aGvHD, one after the conventional 100 d window (d 141). Compared with historical controls, the incidence of aGvHD was significantly reduced for Grade II (p < 0.005) and Grade >III (p = 0.01), using the two-tailed Z-test of proportions.
Experimental Endpoints: Acute GvHD is associated with increased gut permeability, bacterial translocation, and loss of gut microbiome diversity. Endotoxemia, an indicator of intestinal integrity, as measured by the endotoxin activity assay (EAA, Spectral Medical) reached septic levels in 40% of patients during pre-transplant conditioning (d -3 - d 0), and subsequently returned to, or maintained, non-septic levels from week 5 onward in 90% of patients, with an overall downward group trend of EAA scores during 180 days of follow up. Genotyping and analysis of bacterial V4 16S ribosomal DNA from weekly stool samples of the first 10 evaluable patients revealed no decrease in OTU richness or Shannon diversity at any time point pre- vs. post- treatment. Among the 5 most abundant phyla, Bacteroidetes was significantly less abundant post-treatment compared to pre-treatment. Among the 8 most abundant families, Bacteroidaceae significantly decreased post- vs. pre- treatment. While historic microbiome controls from our institution are not available, our finding of increased Bacteroidetes is in contrast to reported decreased Bacteroidetes in allogeneic HSCTs not receiving TMS, but may resemble changes after autologous HSCT.
This trial, funded by the Gateway Foundation, is ongoing. Preliminary results indicate TMS is well tolerated, with a beneficial impact on aGvHD and no increased relapse or NRM, with excellent engraftment, and preservation of anti-tumor effect, intestinal barrier integrity, and gut microbial diversity.
Iyengar:Hackensack University Medical Center: Patents & Royalties: Telmisartan use patent.. Rowley:Allergan: Equity Ownership; Fate Therapeutics: Consultancy. Schwartz:Hackensack University Medical Center: Patents & Royalties: Telmisartan use patent.
Telmisartan. Indicated for treatment of hypertension.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal