Introduction
HCMV infection is a significant viral complication in HSCTR, causing either a subclinical infection or a severe disease (systemic syndrome and localized tissue invasive disease (TID)).
Pre-emptive therapy used for the management of HCMV infection and prevention of disease requires the monitoring of HCMV load in blood to start antiviral therapy after detection of a viral load threshold associated to the risk for HCMV severe infection and disease. In previous studies we observed that patients reconstituting both CD4+ and CD8+ HCMV-specific T cells are generally protected from HCMV disease. Thus, determination of specific T cells may discriminate patients lacking immunity and requiring stricter virological monitoring (negative T-cell response) from those early recovering T-cell immunity and thus not requiring antiviral interventions (positive T-cell response).
Aims
The aim of our work is to assess the possibility to manage HCMV infection by combined virological and immunological monitoring.
The primary endpoint of this study is to compare the frequency of HCMV disease between two groups of HSCTR: in the first one, pre-emptive therapy was started according to combined virological/immunological methods; the second group consisted of an historical cohort of patients monitored with HCMV-DNA as the only guiding parameter for pre-emptive therapy.
Methods
We analyzed adult patients receiving allogeneic HSCT: the recipients, the donor or both had to be HCMV seropositive.
Virological follow-up (HCMV-DNA determination in whole blood) was performed weekly for the first 3 months, then monthly during the first year. Immunological follow-up was performed at 1, 2, 3, 4, 6, 8, 10 and 12 months. Peripheral blood HCMV-specific T cells were determined by two assays: i) iCL (infected Cell Lysate)-ELISPOT, considering as "protective" immunity a blood count of specific T-cell ≥ 0.1/µL ii) iDC-CFC (infected Dendritic Cells-Cytokine Flow Cytometry), considering as "protective" immunity a blood count of CD4+ T-cell ≥ 1/µL and CD8+ T cells ≥ 3/µL.
According to study, within the first 3 months following transplantation pre-emptive antiviral therapy was given to all patients presenting a viral load ≥30.000 copies/mL blood (severe infection). Beyond 3 months following transplantation, patients with negative T-cell response continued receiving a pre-emptive antiviral therapy as reported above. In patients with positive T-cell response confirmed by both assays ("protected" patients), antiviral therapy was deferred even if viral load would reach ≥30.000 copies/mL blood.
Results
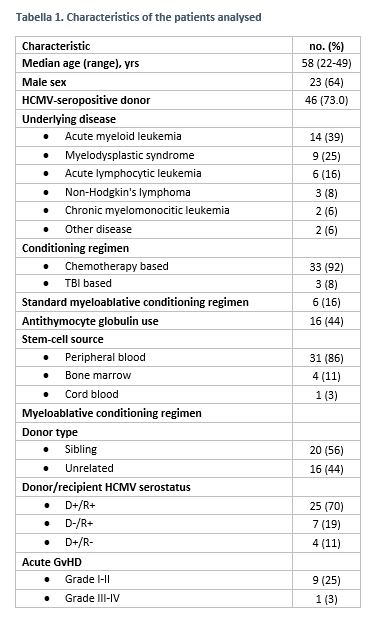
We performed an analysis of 36 patients with a follow-up ≥4 months from HSCT (median follow-up 245 days, 120-397; see Table 1 for patients' characteristics).
Among them, 32 HCMV-seropositive HSCTR developed HCMV infection whereas 4 HCMV-seronegative patient receiving HSCT from seropositive donors did not (nor developed specific immunity). Among the 32 HSCTR with HCMV reactivation, 13 (41%) resolved spontaneously HCMV reactivation, whereas 19 (59%) had a severe infection (11 [34%] controlled the infection after a single course of Valganciclovir therapy and the remaining 8 [25%] required multiple courses for relapsing episodes). Thirty days after HSCT, levels of total and HCMV-specific CD4+ and CD8+ T cells were significantly higher in the 13 patients spontaneously resolving the infection than in the 19 patients with severe infection. The two immunological assays were concordant in detecting time to protective immune reconstitution (median time: 177 vs 183 days for iDC and iCL-ELISPOT, respectively).
HCMV-specific immune reconstitution was significantly delayed in patients with multiple infection episodes (median time: 250 days post-transplantation) vs patients with a single episode of severe infection (187 days) and patients with self-resolving infection (89 days). No patient developed HCMV severe infection after HCMV-specific immune reconstitution. Currently, no patient developed HCMV disease.
Conclusion
These results suggest that combined virological/immunological monitoring can be useful to identify patients not requiring strict virological controls and antiviral interventions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal