Background
Chronic graft-versus-host disease (cGVHD) is the major cause of treatment related morbidity and mortality after allogeneic stem cell transplantation (allo-HSCT). Recently Paul Martin et al. reported that treatment success at 1 year (defined as a patient being in partial (PR) or complete remission (CR) of cGVHD, in ongoing remission of underlying disease and not requiring any secondary treatment for cGVHD) was the only endpoint associated with clinical benefit. Strikingly this robust endpoint was only achieved in less than 20% of patients undergoing primary treatment for cGVHD. Therefore, although most patients show initial response to first-line therapy, long-term clinically meaningful treatment success remains rare.
Methods:
We conducted a prospective multicentre phase II trial in 6 transplant centres across Germany (ClinicalTrials.gov Identifier: NCT01862965). Patients aged 18 years or older with newly diagnosed moderate or severe cGVHD were included. All patients received prednisone (1mg/kg BW) and everolimus (target level 3-8 ug/l). Study treatment was scheduled for 12 months followed by a one-year follow-up period. Primary endpoint was treatment success at 6 months defined as patient being alive, achieving PR or CR of cGVHD, having no relapse of underlying disease and requiring no secondary treatment for cGVHD. Secondary endpoints included time to treatment failure, overall survival at one and 2 years, time to response, incidence of PR /CR at 6 months, incidence of relapse, thrombotic microangiopathy, pneumonitis and osteonecrosis.
Results:
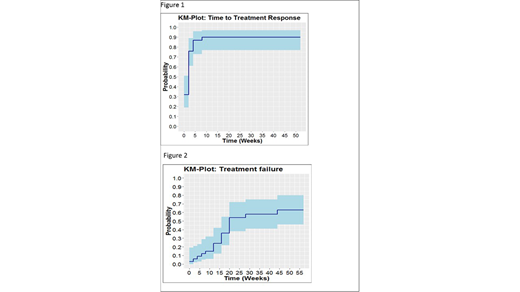
A total of 38 patients were included between March 2013 and February 2018 in the study. Four patients were excluded from the efficacy analysis because of screening failure. Of the 34 patients, 61% had moderate and 39% severe cGVHD. Of these 19 (56%) patients showed treatment success at 6 months with 22% and 52% of the patients in a CR and PR respectively. Overall 30 patients (88%) had a CR or PR as best response, nearly all responses (29/30) occurring within the first 6 weeks of treatment (figure 1). Median time to treatment failure was 24.7 weeks. The cumulative incidence of treatment failure at one year was 63%, resulting in a treatment success incidence of 37% (figure 2). Relapse of underlying disease was diagnosed in 2 (5.9%) patients. Other predefined side effects (thrombotic microangiopathy, pneumonitis and osteonecrosis) were not observed in any patient.
Conclusion:
This, to the best of our knowledge, is the first prospective study analysing the endpoint treatment success as defined by Paul Martin et al. Our results indicate that addition of everolimus to prednisolone is well tolerated and may help improve long-term outcome of patients with cGVHD.
Acknowledgements:
This study was supported by a research grant from Novartis to Nicolaus Kröger and Francis Ayuk
Ayuk:Novartis: Honoraria, Other: Advisory Board, Research Funding. Wagner:MEDAC: Other: Travel support; Novartis: Other: Advisory board; Pfizer: Other: Advisory board; MSD: Other: Advisory board. Wolff:Neovi: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Mallinckrodt: Honoraria. von Harsdorf:Novartis: Other: none. Koenecke:Novartis: Other: none. Sayer:Novartis: Other: none. Kroeger:Novartis: Honoraria, Other: Advisory Board, Travel Costs, Research Funding.
Everolimus used for Treatment of chronic GVHD
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal