Introduction
Lower respiratory tract infections (LRTI) due to parainfluenza (PIF) or respiratory syncytial virus (RSV) represent a major challenge for immunocompromised patients especially those after allogeneic stem cell transplantation (allo-SCT). Evidence-based guidelines for the management of respiratory virus infections in allo-SCT recipients are limited due to the paucity of effective antivirals and the lack of prospective randomized trials. In this study, we report our experience with the use of oral ribavirin with or without the addition of IVIgG in allo-SCT recipients with LTRI due to RSV and PIF.
Patients and methods
This is a retrospective study performed in 3 BMT centers in Athens, Greece (2 adult and 1 pediatric center). Review of medical records was performed with the aim to identify patients with RTIs who received treatment with oral ribavirin. LRTI was defined according to the European Conference on Infections in Leukaemia (ECIL-4) (Ref 1). Presence of RSV or PIF in specimens from nasopharyngeal wash and/or BAL was documented with the use of a PCR assay. Ribavirin was administered at a dose of 20-30mg/kg divided into 4 daily doses, with appropriate dose adjustment according to creatinine clearance. Treatment with ribavirin was continued until significant symptomatic and radiological improvement. Co-administration of IVIgG was at the discretion of the treating physician and was mostly based on the availability of IVIgG and on the severity of underlying illness. IVIgG was administered at a daily dose of 400mg/kg for a total dose of 2 g/kg. Informed consent was given by patients or their parents.
Results
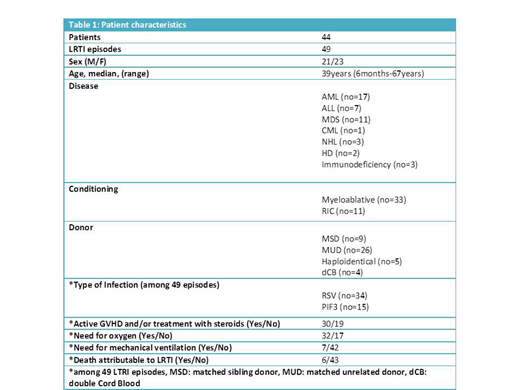
Forty-nine episodes of LRTI due to RSV or PIF were reported during a nine-year period in 7 children and 37 adult patients after allo-SCT. Three children and 2 adult patients had a second LRTI due to RSV, 6, 8, 8, 10 and 12 months after the first episode. Patient's characteristics are shown in Table 1. All patients presented with fever and cough. In almost all of the patients auscultation revealed a prolonged expiratory phase, diffuse wheezing, while the presence of crackles was more prominent finding in patients presenting with pneumonia. Arterial blood gas analysis showed moderate to severe hypoxemia. In 32 out of 49 episodes oxygen supplementation was required while 7 patients required mechanical ventilation. All 49 episodes treated with oral ribavirin while in 37 cases ribavirin was administered in combination with IVIgG.
High resolution CT-scan was performed in 41 episodes and revealed bilateral abnormalities in all patients examined. Small centrilobular nodules, bronchial wall thickening (32 out of 41 episodes) and ground-glass opacities (21 out of 41 episodes) were common findings, while a pattern of diffuse air-space consolidation was observed in 11 cases.
In 43 LRTI episodes a rapid response to treatment in a median of 3 days (range, 2 - 5) was observed. Complete resolution of all symptoms and signs of disease occurred after a median of 9 days (7 - 20) of treatment. One patient with pneumonia due to PIF3 who needed mechanical ventilation had a complete resolution of all symptoms of disease. Six deaths attributable to LRTI were observed among a total of 49 episodes (all 6 patients were in need for mechanical ventilation). Disease relapse confirmed microbiologically occurred in only 1 patient with LRTI due to RSV 10 days after the end of treatment with ribavirin. One children developed bronchiolitis obliterans syndrome (BOS) 9 months after the resolution of RSV infection. Cryptogenic organizing pneumonia (COP) was observed in two patients 1 and 2 months after the complete resolution of LRTI due to PIF. Both patients had an excellent response to treatment with steroids.
Conclusions
Oral Ribavirin has an excellent efficacy and safety profile. Complete and fast resolution of infection occurred in 88% of cases. From the present study it is not clear if the addition of IVIgG offers any therapeutic benefit.
References
Fourth European Conference on Infections in Leukaemia (ECIL-4): Guidelines for Diagnosis and Treatment of Human Respiratory Syncytial Virus, Parainfluenza Virus, Metapneumovirus, Rhinovirus, and Coronavirus. Clin Infect Dis 2013;56(2):258-66
Tsonis:Gilead: Other: Travel Grant; Astellas: Other: Travel Grants; Innovis: Other: Travel Grant; Pfizer: Other: Travel Grant; Takeda: Other: Travel Grant; Aenorasis: Other: Travel Grant.
Tabl Ribavirin. Current indication: Treatment of active hepatitis C in combination with interferon-a.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal