<Introduction>
Delayed immune reconstitution after allogeneic transplantation increases the risk of treatment-related mortality, and chronic GVHD. Previous reports showed that absolute lymphocyte count at day 30 (ALC30) was a significant prognostic factor of transplantation, and lower numbers of total CD4+ T cells and naïve CD4+ T cells in particular were associated significantly with higher mortality. However, there is little knowledge about the factors associated with low lymphocyte recovery, especially in cord blood transplantation (CBT). The cut-off value of lymphocyte recovery for statistical significance has not been determined yet.
<Methods>
We retrospectively analyzed the outcome of 579 consecutive patients who underwent single cord blood transplantation (CBT) for the first time at Toranomon Hospital between January 2011 and 2018. Patients with active infection at transplantation (n=40), in poor ECOG PS (3 or more) (n=36), or lacked information about CT before CBT (n=1) were excluded from this study.
<Results>
Five hundred and two patients (n=317 male; n=185 female) were included in this study. The median age at transplantation was 57 years (range, 16-77), with a median HCT-CI score of 2 (0-10). Underlying diseases were AML (307), MDS (43), MPN (20), ALL (50), mature lymphoid malignancies (54), and others (28). Median spleen index (SI) before transplantation was 60.2 (16.5-319.7). Three hundred and ninety eight patients (79%) were not in remission at transplant. MAC regimens were selected in 400 (80%). TAC alone was used in 132 (26%) as GVHD prophylaxis. Median number of TNC and CD34+ cells infused were 2.62 (1.57-6.85) x 107/kg and 0.86 (0.29-3.77) x 105/kg, respectively. 194 (39%) were positive for anti-HLA antibodies, but none had donor-specific.
With a median follow-up of 32 (range, 3-99) months, cumulative incidence of neutrophil engraftment (NE), the 3-year probabilities of overall survival (OS), relapse rate (RR) and non-relapse mortality (NRM) for entire population were 92.8%, 40.6%, 23.5%, and 35.3%, respectively. Underlying disease (myeloid malignancy), disease status at SCT (non-CR), poor PS (PS=2), GVHD prophylaxis (TAC+MMF), low CD34-positive cell dose (<0.8 x 105/kg), and splenomegary (SI ≥ 40) were significantly associated with inferior NE in multivariate analysis. Disease status at SCT (non-CR), poor PS (PS=2), Higher HCT-CI (PS ≥ 3), and GVHD prophylaxis (TAC+MMF) were significantly associated with higher NRM in multivariate analysis.
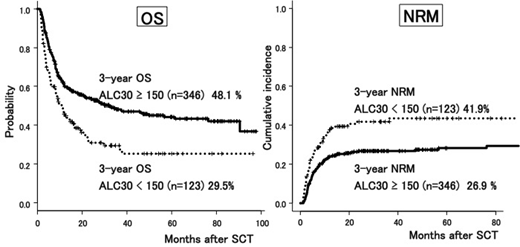
We analyzed the relationship between ALC30 and transplantation outcome. Median number of ALC30 was 231 (3.5-1503), and the 25th percentile was 144. We divided the cohort into two groups, ALC30 low group (<150, n=123), ALC30 high group (≥150, n=346). High number of ALC30 (≥150) was associated with better survival (48.1% vs. 29.5%, p < 0.01) and lower NRM (26.9% vs. 41.9%, p < 0.01) due to reduced incidence of lethal infection (7.1% and 23.4% of total death in ALC30 high and low, respectively, p < 0.01), but was not associated with RR (25.5% vs. 25.1%, p = 0.59). High ALC30 (≥150), as well as younger age (<60), underlying disease (myeloid malignancy), better PS (0-1), and disease status at SCT (CR) showed a superior OS in multivariate analysis. We then assessed factors associated with ALC30, and higher infused dose of CD34-positive cells was the only factor associated with high ALC30, (p=0.03, t-test). Other factors, such as infused TNC dose, type of conditioning, or GVHD prophylaxis did not show significant association with ALC30.
<Conclusion>
This retrospective study demonstrated that low ALC30 after CBT had negative impact on survival because of higher NRM rate. High CD34-positive cell dose was the only factor associated with high ALC30. Not CD34-positive cell dose, but ALC30 had significant impact on OS in multivariate analysis, so we need to take lymphocyte recovery into account, in an attempt to improve transplantation outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal