Background
Although Respiratory Syncytial Virus (RSV) is a commonly recognized respiratory pathogen that can cause upper respiratory tract infections (URTI) and severe lower respiratory tract infections (LRTI) in infants and young children, it can also affect immunocompromised (IC) patients. Hematopoietic cell transplantation (HCT) recipients are at high risk for severe disease caused by RSV. The progression of RSV from URTI to LRTI can cause significant morbidity, often leading to hospitalization, intensive care unit admissions for supportive care and results in mortality in 2-5% of RSV infected HCT recipients.
At some clinical centers oral or intravenous ribavirin, intravenous immunoglobulin or anti-RSV-enriched antibody preparations are used to treat RSV infection. However, there are currently no approved direct-acting antiviral agents indicated for the prevention or treatment of RSV infection in IC adult patients. A recently conducted trial evaluating another RSV fusion inhibitor in HCT recipients demonstrated strong trends toward clinical benefit among patients who were early in their disease course and were severely immunocompromised. JNJ-53718678 (JNJ-8678) is a fusion protein inhibitor that was well tolerated and showed antiviral activity in a Phase 1b study of RSV-infected infants, and was well tolerated and demonstrated antiviral efficacy in healthy adult volunteers in a human viral challenge study. FREESIA will evaluate the clinical outcomes, antiviral activity, safety, tolerability, pharmacokinetics (PK), and PK/pharmacodynamics (PD) of JNJ-8678 in HCT recipients with RSV URTI who are at the highest risk for progression to LRTI.
Study Design and Methods
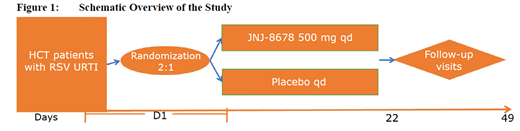
This is a randomized, double-blind, placebo-controlled, multi-center study to evaluate JNJ-8678 in adult and adolescent HCT recipients with RSV URTI. In the adult cohort, a sample size of approximately 249 patients is targeted. (Figure 1).
Adult and adolescent HSCT recipients will be enrolled if they present for medical care with an absolute lymphocyte count <1,000 cells/μL, a PCR-based diagnosis of RSV infection, new onset of at least one respiratory symptom (nasal congestion, rhinorrhea, cough or pharyngitis) and/or worsening of one of these symptoms, if chronic due to an existing diagnosis, within 4 days prior to the anticipated start of dosing and no evidence of abnormalities consistent with LRTI on a chest X-ray. Eligible patients will be randomized 2:1 to receive orally either 500 mg JNJ-8678 once-daily (qd) or placebo qd for 21 days. The follow-up period will be 28 days in duration.
The primary endpoint is the proportion of patients developing RSV LRTI, by external adjudication. Clinical course of RSV infection, antiviral activity, health-related quality of life, RSV viral sequencing and RSV viral kinetics will also be assessed. The PK/PD relationship of JNJ-8678 exposure with selected efficacy and safety parameters will be explored. Safety and tolerability, including AEs, laboratory assessments, ECGs, vital signs, and physical examination will be assessed.
This study will begin recruitment globally in the northern hemisphere in November 2019. This study will clarify the role JNJ-8678 may play in the treatment of RSV in a broader IC population, potentially addressing a substantial unmet medical need.
Chemaly:Gilead: Research Funding; Gilead: Other: Personal fees; Chimerix: Research Funding; ReViral: Other: Personal fees; Oxford Immunotec: Other: Personal fees; Ansun: Other: Personal fees; Chimerix: Other: Personal fees; Oxford Immunotec: Research Funding; Merck: Research Funding; Ansun Pharmaceuticals: Research Funding; Janssen: Other: Personal fees; ADMA Biologics: Other: Personal fees; Merck: Other: Personal fees; Clinigen: Other: Personal fees; Ablynx: Other: Personal fees. Boeckh:Chimerix: Research Funding; GSK: Other: Personal fees; Merck: Research Funding; GSK: Research Funding; Clinigen: Other: Personal fees; Merck: Other: Personal fees. Weber:Janssen Pharmaceuticals: Employment; Johnson & Johnson: Equity Ownership. Fleischhackl:Johnson & Johnson: Equity Ownership; Janssen Pharmaceuticals: Employment. Stevens:Johnson & Johnson: Equity Ownership; Janssen Pharmaceuticals: Employment. Michiels:Johnson & Johnson: Equity Ownership; Janssen Pharmaceuticals: Employment. Rivas:Janssen Pharmaceuticals: Employment; Johnson & Johnson: Equity Ownership. Chien:Johnson & Johnson: Equity Ownership; Janssen Pharmaceuticals: Employment. Witek:Johnson & Johnson: Equity Ownership; Janssen Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal