Introduction:
ABO blood group incompatibility is not a barrier to performing allogeneic stem cell transplant, but may result in life-threatening acute hemolytic reactions as well as pure red cell aplasia. As stem cell product manipulation is cumbersome and may entail cell loss, attempts have been made in the past to reduce IHA titers in-vivo either by donor type red cell transfusion or using frozen plasma in peripheral blood stem cell (PBSC) transplants (Scholl et al. Transfusion 2005; Damodar et al. BMT 2005). The efficacy of such strategies have not been described in Bone marrow transplant (BMT) setting. We are reporting the effectiveness and safety of donor type red cell infusion during conditioning as a method of reducing acute hemolytic reaction during transplant while using unmanipulated marrow as stem cell source (BMT).
Materials and Methods:
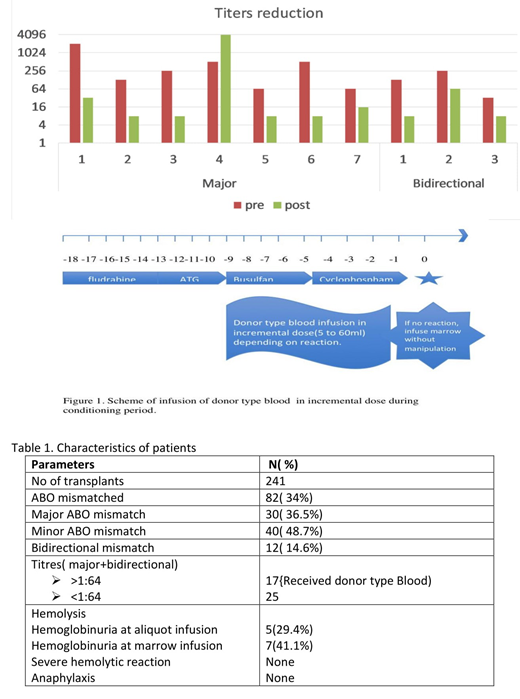
We retrospectively analyzed 241 consecutive allogeneic BMTs for beta thalassemia major, between August 2015 and July 2019 out of which 82 were ABO mismatched transplants, either major (n=30) or minor (n=40), or bidirectional (n =12) mismatched. Infusion of donor type red blood cell during conditioning after the infusion of Anti-thymocyte globulin (ATG) and post-transplant complication of acute hemolysis were determined by retrospective review of individual medical records. When there is a major ABO incompatibility and IHA titers against the donor were > 1:64, a single unit of donor type Packed Red Blood cell (PRBC) was divided into 4 aliquots, irradiated and administered over 4 days at increasing incremental volumes once daily over 4 days if tolerated (Day 1 - 5 ml, Day 2 - 10ml, Day 3- 20-30 ml, Day 4 - 40-60 ml) (Fig.1). Patients were watched carefully for febrile reactions and hemoglobinuria and mild reactions were tolerated. If no clinical evidence of severe hemolytic reaction, bone marrow was infused without manipulation on the day of transplant.
Results:
Out of 30 patients with major ABO incompatibility, 13 patients had titers more than 1:64 (highest was 1:2048) and hence received donor type PRBC infusion in small incremental doses. Eight patients showed evidence of some hemolysis (4 during infusion of donor type PRBC aliquot and 4 showed increase in indirect bilirubin with marrow infusion) which was managed conservatively with hydration. None of the patients developed severe hemolytic or anaphylactic reaction at the time of marrow infusion. Post infusion of donor type blood, titers were checked in 7 patients. 6 patients had significant reduction in titers (all were less than 1:32) except for 1(titers increased to 1:4096), which was not considered clinically relevant as he tolerated 100mls of donor type PRBC. He also tolerated marrow infusion without any evidence of severe hemolytic reaction. Four more patients with bidirectional mismatch had IHA titers against the donor more than 1:64, hence the same procedure was followed. One of them had mild hemoglobinuria during donor type PRBC infusion and 3 patients had mild hemoglobinuria with rise in indirect bilirubin at the time of marrow infusion. All patients were managed conservatively with hydration.
Conclusion:
Our experience demonstrated that donor-type PRBC infusion as a method of in-vivo adsorption of IHA antibodies against donor is safe and effective in preventing acute hemolysis in major ABO-mismatched stem cell transplants even in the context where bone marrow is used as graft source. This simple method in addition to avoiding the problems related to product manipulation can also be safely and easily performed in resource limited settings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal