Background. Allogeneic hematopoietic stem cell transplant (HSCT) is a promising approach to halt disease progression and prevent or ameliorate neurological symptoms arising from select inherited metabolic disorders (IMDs). Donor-derived cells, including microglia, limit disease progression post-HSCT via production of normal enzyme in a process called cross-correction. A standard cell dose used in HSCT is sub-optimal, resulting in delayed hematopoietic recovery and slower correction of central nervous system (CNS) defects (Lund et al BBMT 2019). To address these limitations, we developed MGTA-456, a cell therapy that contains large numbers of CD34+ cells and has led to accelerated neutrophil recovery and 100% engraftment post-HSCT in patients with malignant and non-malignant diseases (Wagner et al Blood 2017; Orchard et al AAN 2019). We previously showed that MGTA-456 leads to faster hematopoietic and microglia recovery in the brains of NSG mice (Goncalves et al AAN 2019); however, the impact of cell dose on disease outcomes and mechanism of cross-correction are unknown. Here, we show that faster and greater hematopoietic and microglia recovery leads to rapid and complete resolution of disease endpoints in a mouse model of mucopolysaccharidosis I (Hurler syndrome) and that, mechanistically, donor engraftment in the brain is required for disease cross-correction.
Results. To determine whether cell dose impacts microglial engraftment, CD45.2 mice were conditioned with a clinically-relevant, myeloablative dose of busulfan and transplanted with increasing doses of CD45.1 bone marrow cells, beginning with 0.3x106 cells/mouse (2x106 cells/kg) based on allometric scaling to model high dose cell therapies. A dose-dependent increase in microglia was observed as early as 1 week post-HSCT, where 10x106 cells led to a 26-fold higher number of donor microglia compared to 0.3x106 cells (p<0.01), an effect that was sustained through 16 weeks post-HSCT (p<0.001). Despite high donor chimerism in the periphery at all cell doses (75-99%), only partial chimerism was observed in the brain. At 16 weeks, donor microglia represented only 2% of microglia after transplant of 0.3x106 cells but this was increased to 35% of total microglia in the brain following transplant of 10x106 cells. These data indicate that while busulfan can facilitate a low level of microglia engraftment, this effect can be enhanced by transplant of high cell doses.
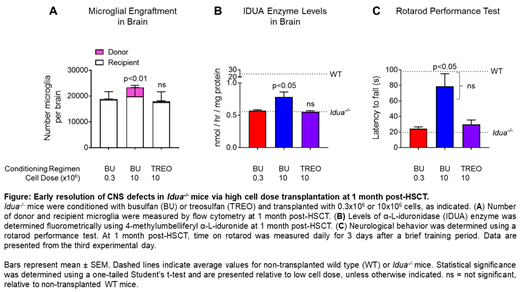
To evaluate the impact of cell dose on disease outcomes, we transplanted a low (0.3x106) or high (10x106) cell dose of wild-type bone marrow cells into busulfan-conditioned Idua-/- mice, a model of Hurler syndrome. At 1 month post-HSCT, peripheral donor myeloid chimerism was >75% and >99% for 0.3x106 and 10x106 cells, respectively. In the brain, transplant of 10x106 cells led to significantly higher donor microglial engraftment versus 0.3x106 cells (Figure A). Notably, high cell dose resulted in significantly higher levels of IDUA enzyme in the brain (Figure B), reduced levels of β-hexosaminidase and glycosaminoglycan (GAG) substrate, and normalization of behavioral outcomes, including rotarod performance, to wild type levels (Figure C). In peripheral tissues, transplant of 10x106 cells, but not 0.3x106 cells, led to a reduction of GAGs to wild type levels as early as 1 week post-HSCT (p<0.01).
To determine if donor engraftment in the brain is required for cross-correction, we transplanted 10x106 cells into mice conditioned with a myeloablative dose of treosulfan, which is not sufficient to condition the brain for microglia engraftment. Treosulfan conditioning, followed by high dose HSCT, led to >99% donor myeloid chimerism in the periphery but neither increased microglial levels nor corrected CNS defects (Figures A-C), suggesting that donor engraftment in the brain is required for disease modification. Long-term outcomes and impact on skeletal phenotype in this model will also be presented.
Conclusions. We demonstrate that high dose HSCT leads to robust microglia engraftment in the brain and improved disease endpoints. These data suggest that strategies to increase cell dose, such as MGTA-456, may accelerate resolution of neurologic disease in patients with IMDs. Similar approaches, possibly coupled with gene modification technologies, could be used to improve microglial function in other neurodegenerative diseases where defective microglia have been implicated.
Goncalves:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Hyzy:Magenta Therapeutics: Employment, Equity Ownership. Brooks:Magenta Therapeutics: Employment, Equity Ownership. Boitano:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Cooke:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal