Introduction:
Systemic light-chain (AL) amyloidosis results from clonal plasma cells that secrete toxic fibril-forming free light chains. Therapies directed at the plasma cell clone form the backbone of its management. Identification of cell-surface receptors on the clonal cells can provide targets for therapy. BCMA is one such cell-surface glycoprotein; it is principally expressed on plasma cells and supports their long-term survival (J Exp Med. 2004;199:91-98). Anti-BCMA immunotherapies are currently being studied in multiple myeloma (N Engl J Med. 2019;380:1726-1737). Membrane-bound BCMA (mBCMA) is also shed as a soluble form, sBCMA, due to γ-secretase activity that can be inhibited by a small molecule (GSI, LY-411575) (Nat Commun. 2015;6:7333; J Immunol. 2017;198(8):3081-3088). We report on mBCMA on the clonal plasma cells of AL patients and its modulation by GSI in vitro, and on sBCMA in the blood of AL patients and of mice xenografted with an AL cell line, demonstrating its correlations in vivo with free light chain (FLC) levels and plasma cell tumor burden.
Methods:
We analyzed mBCMA and sBCMA levels in marrow aspirate and peripheral blood samples from AL patients under an IRB approved protocol. We isolated mononuclear cells (MNC) from patient marrow aspirates with anti-CD138 microbeads (Miltenyi Biotec, Auburn, CA), and used the CD138-selected cells in culture with LY-411575 (Sigma Aldrich, St Louis, MO). We analyzed mBCMA expression by flow cytometry using APC conjugated anti-CD269 (BCMA) antibody (Biolegend, San Diego, CA, USA) and CD138 expression by PE-conjugated anti-CD138 antibody (Biolegend, San Diego, CA, USA), along with appropriate isotype controls. We injected 107 ALMC-1 reporter cells in the flanks of NOD scid gamma (NSG) mice to create a xenograft model of AL clonal plasma cell disease (Jackson Laboratories, Bar Harbor, ME). sBCMA in patients and mice and FLC in mice were measured by ELISA (R&D Systems, Minneapolis, MN; Bethyl lab Montgomery, TX respectively). Pearson and Spearman correlation analysis was used to examine associations of sBCMA and clinical disease parameters. Paired t-test was applied to compare BCMA expression before and after treatment with GSI.
Results:
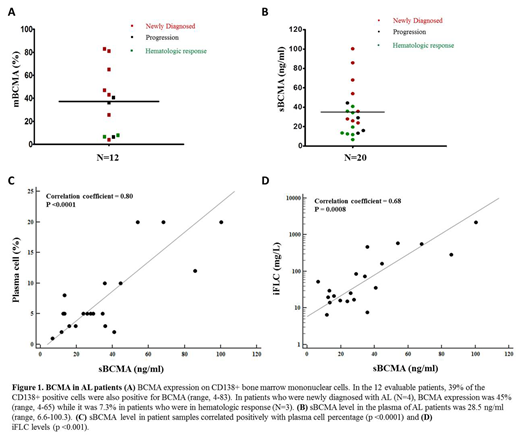
Marrow and blood were obtained from 20 AL patients, 8 newly diagnosed, 4 with progression of disease, and 8 after treatment with >VGPR. Their median age was 65 years (range, 48-77) and 50% were female. Median plasma cells in the marrow aspirates and involved FLC levels were 5% (1-20%) and 33 mg/L (6.6-2220mg/L) respectively. Median mBCMA expression on CD138+ marrow MNC and sBCMA levels in plasma were 39% (4-83) and 28.5 ng/ml (6.6-100.3) respectively (Figure 1A-B). sBCMA levels correlated with bone marrow plasma cell percentage and iFLC (both p<0.001, Figure 1C-D). In culture with LY-411575, the percentages of CD138 cells positive for mBCMA increased from 85% to 100% with ALMC-1 cells and from 36% to 68% (p < 0.01) with patient CD138-selected cells while the sBCMA levels in culture supernatant decreased by over 50%. In NSG mice with ALMC-1 reporter cell xenografts, medians of luciferin-based bioluminescence FLUX (photons/s), λ FLC and sBCMA were 3.9x1010 (2.02x109-1.2x1011), 949.1 mg/L (868.8-23629.2), and 3.8 ng/ml (0.9-23.6) respectively. sBCMA levels correlated with FLC (Pearson r= 0.99, p<0.0001) and with FLUX (Pearson r=0.61, p=0.07).
Conclusions:
BCMA is expressed on AL plasma cells and sBCMA is detected in the blood of all AL patients. In this light chain disease, sBCMA may be useful as a marker of disease activity even in patients with low FLC. Furthermore, expression of mBCMA can be manipulated by treatment with a GSI, an approach which may be useful therapeutically in AL. These results provide the basis for applying anti-BCMA immunotherapies in clinical trials in relapsed refractory AL patients.
Comenzo:Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees; Unum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; Caelum: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Prothena Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Myself: Patents & Royalties: Patent 9593332, Pending 20170008966.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal