Background:
Recent attempts have focused on identifying fewer magnitude of minimal residual disease (MRD) rather than exploring the biological and genetic features of the residual plasma cells (PCs). Interphase fluorescence in situ hybridization (iFISH) analyses in sequential samples provide a simple and reliable method to longitudinal track the dynamic changes in clonal architecture and speculate the possible evolutionary pattern. Here, we report for the first time the incidence and prognostic significance of cytogenetic abnormalities (CA) existed in the PCs of patients achieving at least partial remission.
Methods:
A cohort of 193 patients with at least one CA at diagnosis were analyzed using data from the prospective, non-randomized clinical trial (BDH 2008/02), and iFISH analyses were performed in patient-paired diagnostic and post-therapy samples.
Results:
Persistent CA in residual tumor cells were observed for the majority of patients (63%), even detectable in 28/63 (44%) patients with MRD negativity (<10-4). The absence of CA in residual PCs was associated with prolonged survival regardless of MRD status. It was noted that MRD-positive but FISH-negative patients experienced similar survival to MRD-negative patients (m-TTP 4.5 vs. 5.1 years, P=0.983).
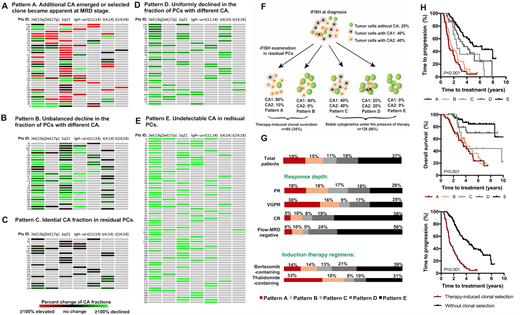
According to the change of the clonal size of specific CA, patients were clustered into five groups, reflecting five patterns of clone selection under therapy pressure. 1) Pattern A were observed in 36 (19%) patients where a minor subpopulation or undetectable subclone in the pre-treatment sample became dominant after therapy. 2) Pattern B was identified in 29 (15%) patients whose fractions of PCs harboring different CA were decreased with inconsistent extent. 3) Identical CA fraction in residual PCs were found in 22 (11%) patients as Pattern C. 4) Pattern D. The fractions of PCs harboring specific CA in 35 patients (18%) were uniformly declined. 5) 71 patients lost their abnormal cytogenetic clone after therapy (less than cut-off level) were classified as Pattern E. The cytogenetic dynamics of pattern A and B can be interpreted as a therapy-induced selection process with comparable inferior survival (m-TTP 1.2 vs. 1.6 years, respectively). Patients with pattern E experienced the most favorable outcome (m-TTP 5.0 years), following those with pattern D and C (m-TTP 3.5 and 2.5 years).
Among the 65 patients with clonal selection, 24 underwent upfront auto-transplantation that experienced significantly improved survival. However, upfront transplant failed to completely reverse the inferior outcome caused by therapy-induced clonal selection. Longitudinal cytogenetic studies at relapse were available in 43 patients. The results suggested that sequential cytogenetic dynamics were observed in most patients, and the cytogenetic architecture of residual cells could to some extent predict the evolutional pattern at relapse.
Conclusions:
The repeat cytogenetic evaluation in residual cells could not only serves as a good complementary tool for MRD detection, but also provides a better understanding of clinical response and clonal evolution. Therapy-induced clonal selection was associated with inferior outcome regardless of the baseline cytogenetic profiles. The early identification of resistant clone may contribute to guide better tailored therapy strategies based on the feature of the residual tumor cells.
Munshi:Celgene: Consultancy; Adaptive: Consultancy; Oncopep: Consultancy; Amgen: Consultancy; Janssen: Consultancy; Takeda: Consultancy; Abbvie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal