The myeloid system has often been regarded as the 'dumb brute' side of cell-based immunity with limited specificity and variability of responses. Heterogeneity in innate immune cells is increasingly-recognized but still modest compared to other cell types and constrained by limited investigative tools. Here, we show that myeloid cells have inherent, restricted capabilities that can be defined and potentially exploited for the development of myeloid cell-based therapies.
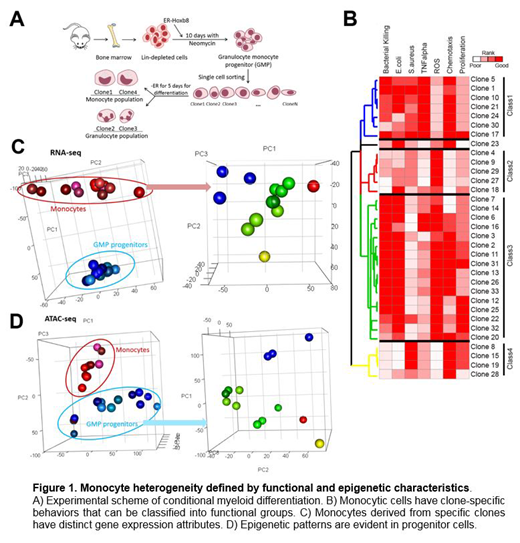
To study myeloid heterogeneity in vitro, we adapted a system for inducible clonal expansion of primary mouse granulocyte-monocyte progenitors (GMP) capable of differentiating into mature myeloid cells (Sykes et al., Cell, 2016). This system allows for the generation of large numbers of primary self-renewing GMP that can undergo progressive maturation to fully functional granulocytes or monocytes upon removal of an inducing agent (Fig. 1A).
By isolating individual self-renewing GMP, clone-specific behaviors and genetic characterization can be tested and repeatedly evaluated. We isolated individual GMP that were capable of maturing into monocytes in vitro and tested whether their functional features were distinctive and reproducible on a clonal level. Functional capabilities that were quantitated include: 1. phagocytosis, 2. bacterial killing, 3. reactive oxygen species generation, 4. TNF-alpha production, 5. proliferation, and 6. cell surface marker production.
Unsupervised clustering of clone-specific monocytic cells defined four different functional groups with distinctive gene expression programs (Fig. 1B). Surprisingly, this clustering is evident in chromatin accessibility at the GMP level without apparent transcriptomic alterations (Figs. 1C, D). Subgroups of monocytes injected into recipient mice, differentially trafficked to and resided in tissues such as bone marrow, spleen, and peritoneum. Furthermore, clones competent in phagocytizing E.coli (gram-negative) versus S.aureus (gram-positive) in vitro demonstrated differential functional ability to reduce peritoneal bacteria burden when adoptively transferred into animals with induced peritonitis.
Molecular characterization of the functional subgroups defined novel subset-specific cell surface protein expression signatures. Antibody combinations reflecting each of these subgroups were applied to primary blood monocytes and enabled prospective isolation of endogenous cells representing each subset. Upon isolation, the mouse primary subset had specialized functional capabilities in vitro and in vivo comparable to the characteristics defined from the in vitro GMP system. Further, human monocyte populations in the blood could be isolated based on the parameters derived from our model and confirmed to have distinctive functional attributes.
These data strongly suggest that diversity among monocytic subsets exists at baseline rather than monocytes transitioning between cellular states in response to particular stimuli. The subset-specific features appear to be established and epigenetically constrained by the time cells have achieved the level of GMP. By defining monocytic subsets with distinctive roles in disease settings, targeting or adoptively transferring them may be of therapeutic benefit.
Sykes:Clear Creek Bio: Equity Ownership, Other: Co-Founder. Scadden:Clear Creek Bio: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Fog Pharma: Consultancy; Red Oak Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; LifeVaultBio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Bone Therapeutics: Consultancy; Novartis: Other: Sponsored research; Editas Medicine: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Consultancy, Equity Ownership; Agios Pharmaceuticals: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal