Background: AL amyloidosis is a bone marrow disorder in which clonal plasma cells produce light chains that misfold and deposit in vital organs, such as the kidneys and heart, leading to organ failure and eventual death. Treatment is directed towards the clonal plasma cell population in an effort to halt the production of toxic light chains and recuperate organ function. Pallidini et al. demonstrated that almost 50% of patients with AL amyloidosis who achieved a complete hematologic response to prior therapy had minimal residual disease (MRD) detectable in their bone marrow by multiparametric flow cytometry (MPF).1. Next generation gene sequencing (NGS) has been a successful tool in measuring MRD among patients with multiple myeloma2 though the data regarding its use in AL amyloidosis are limited. AL amyloidosis is a disease with a much smaller plasma cell burden at baseline (typically 5-10%), making the task of isolating an initial clonal sequence even more challenging. We sought to evaluate NGS as a method of isolating a clonal population of plasma cells among patients with systemic AL amyloidosis in a first-ever feasibility study.
Methods: Patients were eligible if they had systemic AL amyloidosis and no clinical evidence of concurrent active multiple myeloma. In this study, feasibility was deemed successful if discovery of a clone could be achieved in 3 out of 10 of patients. Approximately five cc's of peripheral blood and bone marrow aspirate were collected from each patient and processed for CD138 selection and DNA isolation/purification. De-identified samples were sent to Adaptive Biotech Inc. (Seattle, WA) for initial clonal identification using the ClonoSEQ immunoglobulin heavy chain (IGH) assay. Genomic DNA was amplified by implementing consensus primers targeting the IGH complete (IGH-VDJH) locus, IGH incomplete (IGH-DJH) locus, immunoglobulin κ locus (IGK) and immunoglobulin l locus (IGL). The amplified product was sequenced and a clone identified based on frequency. After proof of feasibility in the first 10 patients an additional 27 patients had initial clonal identification via the same process mentioned above.
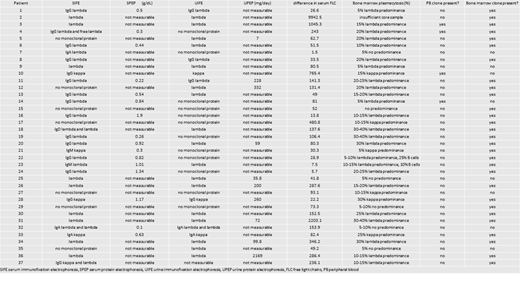
Results: In total, 37 patient samples underwent NGS via the ClonoSEQ IGH assay method. The median patient age was 66 years old (range: 44 to 83), 24% of which were female. All 37 patients had measurable disease based on serum electrophoresis and immunofixation and/or serum free light chain assay (Table 1). Four patients had no monoclonal protein detected on SIFE or UIFE and 13 patients had a normal sFLC ratio. Of the 33 patients with monoclonal disease on immunofixation, 12 patients had only a free lambda monoclonal protein and the remaining 21 patients had a clonal heavy chain with an associated light chain. Bone marrow biopsies demonstrated clonal plasmacytosis of 40% or lower. ClonoSEQ IGH assay identified trackable clones in 31 of 37 patients (84%) (see Table 1). Four patients had at least one trackable sequence (range: 1 to 5 sequences) in the peripheral blood and 29 patients had at least one trackable sequence in the bone marrow aspirate (range: 1 to 7 sequences). No correlation was seen between the detection of a clone and standard measures of plasma cell tumor burden (SIFE, SPEP, UIFE, UPEP, and sFLCs).
Conclusion: NGS was successful in identifying an initial clone in 29 of 37 patients with systemic AL amyloidosis, four of which were detectable in the peripheral blood. Due to the low clonal burden in patients with AL amyloidosis, it is often difficult to assess disease status, especially post-treatment. These encouraging results may enhance disease monitoring and improve patient care in this rare disease. We are currently tracking MRD in the patients with identifiable clones as they receive systemic treatment, the results of which will be available for presentation in December 2019.
REFERENCES
1. Palladini G, Massa M, Basset M, Russo F, Milani P, Foli A, et al. Persistence of Minimal Residual Disease By Multiparameter Flow Cytometry Can Hinder Recovery of Organ Damage in Patients with AL Amyloidosis Otherwise in Complete Response. Abstr 3261. 2016;
2. Ladetto M, Brüggemann M, Monitillo L, Ferrero S, Pepin F, Drandi D, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia. 2014;28:1299-307.
Sarosiek:Acrotech: Research Funding. Sanchorawala:Proclara: Consultancy, Honoraria; Takeda: Research Funding; Caelum: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Prothena: Research Funding; Celgene: Research Funding. Jacob:Adaptive Biotechnologies: Employment, Other: shareholder. Munshi:Amgen: Consultancy; Adaptive: Consultancy; Celgene: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Janssen: Consultancy; Takeda: Consultancy; Takeda: Consultancy; Oncopep: Consultancy; Oncopep: Consultancy; Amgen: Consultancy; Abbvie: Consultancy; Abbvie: Consultancy; Adaptive: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal