Background
For most Multiple Myeloma (MM) patients, M-protein is the gold standard biomarker for disease diagnosis and monitoring. Traditional methods such as serum protein electrophoresis (SPEP) and serum immunofixation electrophoresis (IFE) lack the sensitivity to detect M-protein in patients with minimal residual disease. At ASH 2018, we reported a Mass Spectrometry (MS)-based highly sensitive assay (REmAb) for detecting and quantifying M-protein. This assay exploits the uniqueness of the M-protein sequence and can detect 1 M-protein in 10,000 polyclonal IgG. The current study utilized this established assay to first sequence then monitor M-protein in sera from diagnosis through treatment for patients from the first MCRN-001 Canadian national trial with bortezomib (BTZ)-based pre-induction (PI), augmented high-dose chemotherapy with busulfan + melphalan (BuMel) before ASCT and lenalidomide (Len) maintenance post-ASCT.
Methods
MCRN-001 trial patients were first induced with BTZ before harvesting stem cells. Eligible MM patients received BuMel prior to ASCT. Busulfan was administered IV at 3.2 mg/kg on days -5 to -3, or days -6 to -4 days pre-ASCT (Day 0) and melphalan was given at 140 mg/m2 on day -2 or -3 pre-ASCT. Len administration began 100 days post-ASCT at 10 mg/d (increased when appropriate to 15 mg/d) and continued until progressive disease (PD) onset. IMWG criteria was used to monitor clinical response and PD. 78 patients enrolled in this trial; only 58 offered consent for M-protein monitoring with MS. Serum samples were acquired at time of pre-induction (PI), before ASCT (screening sample), post-ASCT on day 100, every 3 months for the first year and then every 6 months until PD. The M-protein sequence was determined with the REmAb protein sequencing platform from either PI or screening samples when the PI sample was unavailable. For M-protein quantification, each sample was digested with trypsin prior to analysis by liquid chromatography tandem MS with an Orbitrap Fusion Tribrid or Q-Exactive instrument. Patient-specific, unique tryptic peptides, with at least one peptide from each Heavy and Light chain, were targeted by the MS assay. To monitor changes in M-protein levels per patient post-diagnosis and through treatment to complete remission (CR) and/or PD, this study used MS peak areas of patient-specific unique peptides normalized against peak areas of human serum albumin or spiked-in standard peptides.
Results
M-protein sequencing
In this study, the lowest M-protein serum concentration required for sequencing was 0.2 g/dL. 48 out of 58 (83%) patient-specific M-proteins were sequenced from serum. 5 out of 48 patients sequenced discontinued the study early and were excluded from further analysis.
M-protein monitoring
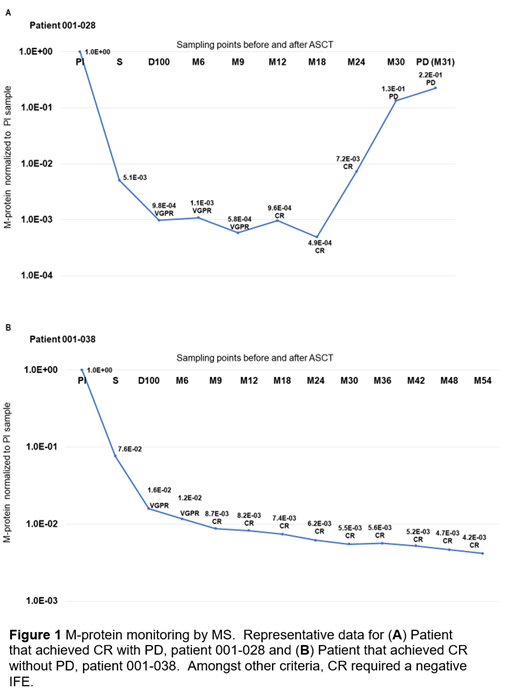
24 out of the 43 achieved CR. The M-proteins of these patients were monitored by MS to study early relapse detection. M-protein levels could be monitored by MS in all these patients from diagnosis through CR to PD onset, even when M-protein was undetectable by SPEP and IFE. A separate serial dilution test estimated that the limit of quantification can reach as low as 0.03 mg/dL. 3 patients who had achieved CR eventually relapsed (PD). In all 3 PD patients, a 2 to 200 fold increase in M-protein in 2 consecutive tests half a year apart was detected by MS 6 months earlier than clinical confirmation of PD by conventional testing. The other 21 had not progressed at time of analysis. In the non-progressor group, the M-protein levels detected by MS either continued to decrease during CR or remained relatively stable. Only 3 out of 21 patients demonstrated a 2-fold increase in M-protein level in any 2 consecutive tests. Figure 1 shows representative data for two patients.
Conclusions
This study demonstrates that M-protein sequencing and targeted MS assay can be used to monitor serum M-protein levels sensitively even when SPEP and IFE fail to detect M-protein. Based on this assay, a 2-fold increase in serum M-protein levels in the past 6 months can reliably predict disease progression in the next 6 months for patients achieving CR. In the 24 studied patients, the method has 100% sensitivity and 86% specificity at predicting disease progression from CR prior to detection by standard methods. Future work will analyze more patient samples to confirm the findings and investigation into criteria surrounding the 2-fold M-protein increase observed in CR patients and relapse post CR.
McDonald:Rapid Novor Inc Kitchener: Employment, Equity Ownership, Research Funding. Liyasova:Rapid Novor Inc Kitchener: Employment. Taylor:Rapid Novor Inc Kitchener: Employment. Xu:Rapid Novor Inc Kitchener: Employment. Gorospe:Rapid Novor Inc Kitchener: Employment. Yao:Rapid Novor Inc Kitchener: Employment. Liu:Rapid Novor Inc Kitchener: Employment, Equity Ownership. Yang:Rapid Novor Inc Kitchener: Employment. Ma:Rapid Novor Inc Kitchener: Equity Ownership. Reece:Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; Merck: Research Funding; BMS: Research Funding. Trudel:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Genentech: Research Funding; Sanofi: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria; Pfizer: Honoraria; Janssen: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
The use of IV busulfan and melphalan as conditioning for myeloma transplants is off-label.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal