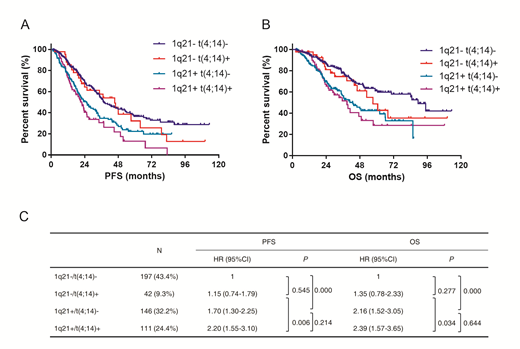
In the era of bortezomib, traditional prognostic markers in multiple myeloma (MM) may be suspect. The outcome of patients with t(4;14) is largely improved by bortezomib based treatment. Whether the outcome of patients with t(4;14) is still homogeneously inferior remains unknown. In this long follow-up non-randomized clinical trial, we observed no significant difference in the PFS and OS of t(4;14) positive or negative patients in the bortezomib arm. Interestingly, t(4;14) was highly correlated with the presence of 1q21 gain. In t(4;14)-positive patients, the median OS of patients with and without 1q21 gain was 38.8 and 57.2 months (P=0.034), respectively. In t(4;14)-negative patients, the median OS of the two subgroups was 43.0 and 89.4 months (P<0.001), respectively. In contrast, no statistical difference in PFS or OS was observed in 1q21 gain patients with versus without t(4;14) aberration. We then discovered that 1q21 gain facilitated the acquisition of new chromosome abnormalities and contributed to the genomic instability, evidenced by the strong correlation between 1q21 gain and complex karyotypes or the acquisition of more than two cytogenetic aberrations. Moreover, 1q21 gain and/or del(17p) were powerful enough to discriminate the high-risk patients. The combination of 1q21 gain with International Staging System (ISS) was useful to divide MM patients with respect to their survival. Finally, although bortezomib might benefit patients with 1q21 gain, it could not completely overcome its adverse effects, suggesting the necessity of more effective therapies for these patients.
Zhan:BIPHARM LLC: Consultancy, Other: % Allocation of Profit. Anderson:Gilead Sciences: Other: Advisory Board; Janssen: Other: Advisory Board; OncoPep: Other: Scientific founder ; Sanofi-Aventis: Other: Advisory Board; C4 Therapeutics: Other: Scientific founder .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal