Background: Bendamustine+rituximab (BR) is an effective chemoimmunotherapy for newly diagnosed chronic lymphocytic leukemia (CLL) and is the most commonly used chemoimmunotherapy in CLL patients requiring therapy (Seymour et al. Cancer. 2019). In a phase III study, the anti-CD20 antibody obinutuzumab (G) showed improved survival in combination with chlorambucil (clb) compared with rituximab (R)+clb, suggesting that G may be a better anti-CD20 monoclonal antibody to combine with bendamustine (B). Here, we report the final study results for the phase II, single-arm, open-label GIBB study (NCT02320487), in which the combination of bendamustine+obinutuzumab (BG) was evaluated in previously untreated patients with CLL. This is also one of the first studies to characterize the evolution of minimal residual disease (MRD) status over time in previously untreated CLL patients receiving chemoimmunotherapy.
Methods: Newly diagnosed patients requiring treatment for CLL had ECOG PS 0-2, and adequate cell counts and organ function. BG treatment was given IV over six 28-day (d) cycles and included obinutuzumab (cycle 1: 100 mg d1, 900 mg d2, and 1000 mg on d8 and d15, and cycles 2-6: 1000 mg d1 of each cycle) plus bendamustine (90 mg/m2 cycle 1: d2 and d3; and cycles 2-6: d1 and d2). The primary end point was complete response (CR), including CR and incomplete CR (CRi), per 2008 iwCLL guidelines after induction treatment. Secondary end points included progression-free survival (PFS) and overall survival (OS). MRD negativity (MRD−) was defined as < 1 cell per 10,000 leukocytes (ie, 10−4) and was measured at baseline, end of treatment (28 d after cycle 6), clinical response assessment, and every 6 months thereafter for ≤ 2 years.
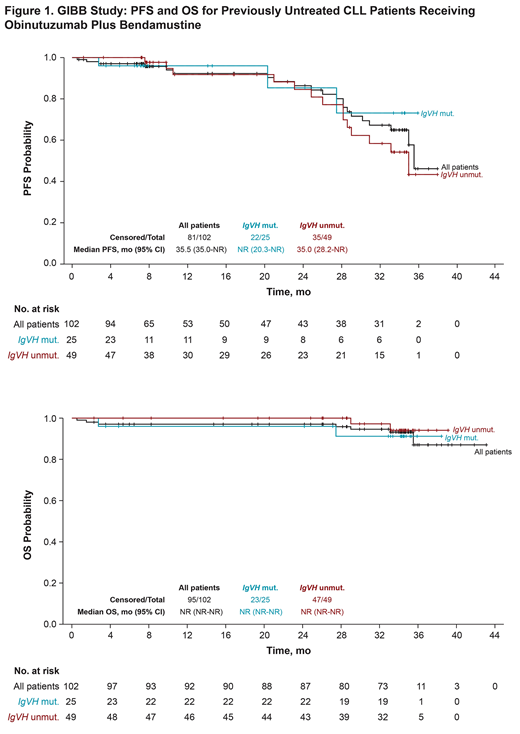
Results: Overall, 102 patients were enrolled on this study. Patients had a median age of 61 years (range, 35-90), and 97% had ECOG PS of 0-1. Of 74 patients evaluated for IgVH status, 49 patients (66%) had unmutated IgVH. Overall, 79% of patients completed 6 cycles of BG. 50% of patients achieved a CR/CRi (95% CI, 40%-60%), which contributed to an 89% overall response rate (95% CI, 82%-95%). At a median follow-up of 34.3 mo, median PFS was 35.5 mo (95% CI, 35.0 mo to not reached) and median OS was not reached (Figure 1). Seven patients died during the study (3 from cardiac events not attributed to treatment by investigators and 4 from other non-progressive disease causes). The most common grade 3/4 treatment-emergent adverse events (> 5%) were neutropenia (25%), infusion-related reactions (9%), anemia (8%), thrombocytopenia (8%), pneumonia (6%), and tumor lysis syndrome (6%). The rate of grade 3/4 febrile neutropenia was 5%. Achievement of MRD− in evaluable peripheral blood and bone marrow at the clinical response assessment was shown in 33/74 (45%; 95% CI, 33%-57%) and 30/51 (59%; 95% CI, 44%-72%) patients, respectively. In the 79 patients (77%) who achieved MRD− in peripheral blood throughout the study, the median duration of MRD− response was 28.9 mo (95% CI, 23.0 to not reached). Of these 79 MRD− patients, 28 became MRD+, and the median time from identification of MRD+ status to progressive disease/death was 10.5 mo (95% CI, 6.3-17.9). An exploratory Cox proportional multivariate analysis identified 2 factors that were significantly associated with longer durations of MRD− responses: mutated (vs unmutated) IgVH with a HR = 0.13 (95% CI, 0.03-0.61), and CD38 ≥ 30% (vs < 30%) with a HR = 0.35 (95% CI, 0.14-0.92). When stratified by IgVH status, the PFS distributions for IgVH mutated and unmutated groups were similar (Figure 1). Median OS was not reached in all patients and irrespective of IgVH status.
Conclusions: Final results for the GIBB study showed that the BG combination was an effective regimen for previously untreated patients with CLL, with no unexpected safety signals. Complete response and MRD− in peripheral blood were achieved in approximately half of all patients after induction treatment and persisted over time. IgVH mutated status was associated with a longer duration of MRD− and median PFS. In summary, BG is a clinically active CLL regimen with durable MRD− rates over time; overall outcomes are consistent with other firstline studies of anti-CD20 antibody and bendamustine combinations.
Sharman:Janssen: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; TG Therapeutics: Consultancy, Honoraria, Research Funding; Acerta: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding. Burke:Celgene: Consultancy; Gilead: Consultancy; Roche/Genentech: Consultancy. Yimer:Amgen: Consultancy; Puma Biotechnology: Equity Ownership; Clovis Oncology: Equity Ownership; Celgene: Honoraria; Seattle Genetics: Honoraria; Janssen: Speakers Bureau; AstraZeneca: Speakers Bureau. Boxer:Rigel: Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Arizona Oncology: Employment; Incyte: Speakers Bureau; Gerson Lerman: Consultancy; Best Doctors: Consultancy; Abbvie: Honoraria, Speakers Bureau. Babu:Fort Wayne Medical Oncology & Hematology: Employment; Bristol Myers Squibb: Consultancy, Research Funding; Fort Wayne Medical Oncology & Hematology: Equity Ownership; Abbvie: Research Funding; Eli Lilly: Honoraria, Research Funding; Pfizer: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Alexion: Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; Incyte: Research Funding; AstraZeneca: Honoraria; Lutheran Hospital, Fort Wayne, Indiana: Membership on an entity's Board of Directors or advisory committees. Li:Roche: Equity Ownership; Genentech: Employment. Mun:Genentech: Employment, Equity Ownership. Danilov:Curis: Consultancy; Verastem Oncology: Consultancy, Other: Travel Reimbursement , Research Funding; Abbvie: Consultancy; Bristol-Meyers Squibb: Research Funding; MEI: Research Funding; Seattle Genetics: Consultancy; Janssen: Consultancy; Aptose Biosciences: Research Funding; Takeda Oncology: Research Funding; AstraZeneca: Consultancy, Research Funding; Pharmacyclics: Consultancy; Gilead Sciences: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; TG Therapeutics: Consultancy; Bayer Oncology: Consultancy, Research Funding; Celgene: Consultancy.
Yes, this was an investigational clinical study of the combination therapy of obinutuzumab and bendamustine in previously untreated patients with chronic lymphocytic leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal