Introduction
The effect of adherence to treatment guidelines has not yet been evaluated in myelodysplastic syndromes (MDS). Based on the large Düsseldorf MDS Registry, we explored adherence to European LeukemiaNet (ELN) guidelines as well as MDS expert recommendations in clinical routine.
Methods
In a preliminary retrospective analysis of 1658 patients documented in the Düsseldorf MDS registry, we reviewed if treatment was in accordance with the published ELN guidelines from 2014. The following treatments were considered: erythropoiesis stimulating agents (ESA), iron chelation, Lenalidomide, hypomethylating agents (HMA), intensive chemotherapy, and allogeneic stem cell transplantation (alloSCT). Since patients were diagnosed between 1982 and 2018, clinical decision-making could not be exclusively based on current guidelines. Therefore, we also performed a prospective analysis of 381 patients who received patient-tailored treatment recommendations, considering, IPSS-R, MDS-CI, HCT-CI, distance to our center, and ELN guidelines, with special attention to the above-mentioned treatment options. In addition, we conducted a matched-pair analysis, comparing patients who received a certain treatment with patients who, though eligible, did not receive it. Information regarding adherence to treatment recommendations was obtained by searching medical files and contacting primary care physicians. Probability of survival was estimated using the Kaplan-Meier method.
Results
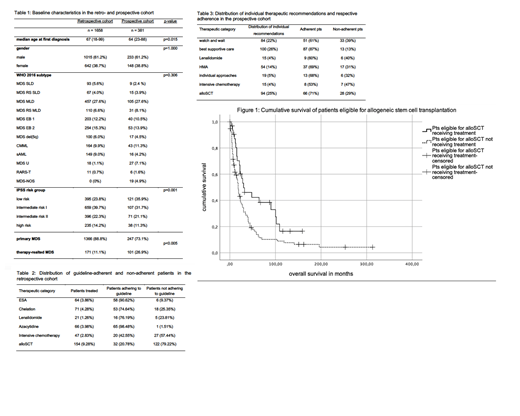
The retrospective cohort was followed for a median of 22 months (1-500 months). Patients treated in accordance with the ELN guideline did not gain a significant survival benefit in the subgroups treated with ESA, iron chelation, Lenalidomide, HMA, and intensive chemotherapy, compared to patients who were eligible for the respective treatment but did not receive it. Solely the group of patients undergoing alloSCT derived a significant survival benefit compared to patients not receiving alloSCT despite qualifying for this treatment according to ELN criteria (30 vs 18 months, p=0.011, 95% CI 16.86; 25.14, fig. 1).
The prospective cohort was followed for a median of 14 months (1-195 months). A median of four months elapsed before treatment was started. Non-adherence to patient-tailored therapeutic recommendations was found in 33% of the patients. The proportion of non-adherence was higher for intensive chemotherapy (47%) and lowest for patients receiving supportive treatment (13%) like ESA and chelation therapy.
In the prospective group, the matched-pair study showed again that patients receiving ESA, iron chelation, Lenalidomide, HMA or intensive chemotherapy did not gain a significant survival benefit from adherence to the respective treatment recommendations. Again, survival only differed between patients adhering to and patients not adhering to a recommendation for alloSCT (median survival of 74 vs 28 months; 95% CI 7.5; 48.5, p=0.015).
Conclusions
According to current ELN guidelines, 33% of patients did not receive the treatment they were eligible for. Non-adherence to patient-tailored treatment recommendations from our tertiary referral center was found in 33% of the patients. Based on our two patient cohorts, supportive care regimens, Lenalidomide, HMA, as well as intensive chemotherapy do not appear to improve overall survival, whereas allogeneic stem cell transplantation provided a significant survival benefit in both groups.
In summary, while adherence to MDS treatment guidelines and expert recommendations may influence survival in some of the patients, the overall effect appears to be limited, owing to the limited efficacy of the available treatment options, with the notable exception of alloSCT. However, we cannot exclude that adherence to treatment recommendations has an effect on other relevant outcomes such as quality of life or frequency of hospitalisations due to infections, bleeding and cardiovascular events.
Finally, a systematic and standardized assessment of guideline-adherence, reasons for non-adherence, and impact on relevant outcomes would be desirable because it would support us in monitoring the quality of care of MDS patients and would allow comparisons to be made between different health economic environments.
Nachtkamp:Jazz Pharmaceuticals: Honoraria; Celgene: Other: Travel Support. Schroeder:Celgene Corporation: Consultancy, Honoraria, Research Funding. Kobbe:Amgen: Honoraria, Other: Travel support, Research Funding; Neovii: Honoraria, Other: Travel support; Abbvie: Honoraria, Other: Travel support; Roche: Honoraria, Other: Travel support; Biotest: Honoraria, Other: Travel support; Celgene: Honoraria, Other: Travel support, Research Funding; Pfizer: Honoraria, Other: Travel support; Jazz: Honoraria, Other: Travel support; Takeda: Honoraria, Other: Travel support; Medac: Honoraria, Other: Travel support; MSD: Honoraria, Other: Travel support; Novartis: Honoraria, Other: Travel support. Kündgen:Otsuka: Honoraria; Takeda: Honoraria, Other: Travel Support; Novartis: Honoraria. Kaivers:Jazz Pharmaceuticals: Other: Travel Support. Rautenberg:Jazz Pharmaceuticals: Other: Travel Support; Celgene: Honoraria, Other: Travel Support. Gattermann:Novartis: Honoraria; Alexion: Research Funding; Takeda: Research Funding. Bonadies:Novartis: Other: financial support for travel, Research Funding; Roche: Other: financial support for travel, Research Funding; Amgen: Other: financial support for travel; Celgene: Other: financial support for travel, Research Funding; Sanofi Genzyme: Other: financial support for travel; Janssen: Other: financial support for travel. Germing:Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal