Background: MDS is characterized by ineffective hematopoiesis and multiple cytopenias. Azacitidine (AZA), a hypomethylating agent (HMA), the standard therapy for higher-risk MDS patients (pts), improves hematopoiesis in 50% of MDS pts, with a median response of 14-24 months. Those pts who initially respond to AZA either relapse or progress with bone marrow failure and have a median survival of 4 to 6 months. Both primary and secondary resistance is a significant challenge and results in poor survival. Rigosertib (RIGO), a small molecule Ras mimetic, as a single agent improved hematopoiesis in 15% of MDS pts who had failed a prior HMA. In vitro data of synergy of RIGO combined with AZA that was sequence dependent (Skiddan et al. AACR 2006), led to a Phase I/II study of the combination of RIGO/AZA and demonstrated an overall response rate of 90% in HMA naïve and 54% in HMA failures pts (Navada et al. ASH 2018). Restoration of functional hematopoiesis in response to treatment with AZA when combined with RIGO in pts, who had failed an HMA, is a unique observation in overcoming the HMA clinical resistance phenotype. Elucidating mechanisms leading to restoration of HMA effects on hematopoiesis could have profound clinical benefit.
Methods: We investigated the molecular mechanisms in response to AZA and RIGO either alone or in sequential combinations (SC) in vitro. Pathway specific real time PCR (QPCR) for epigenetic modification genes, hematopoiesis signaling genes, interferon signaling genes, MAPK signaling genes and antiviral response genes was performed in MDS-L (AZA sensitive) as well asBW-90 (AZA resistant) cell lines treated with RIGO, AZA and SC. Functional analysis was performed using Ingenuity pathway analysis (IPA) software. Reverse phase protein array (RPPA) was also performed on the MDS-L and BW-90 cell lines treated with AZA and RIGO alone and SCs for the validation.
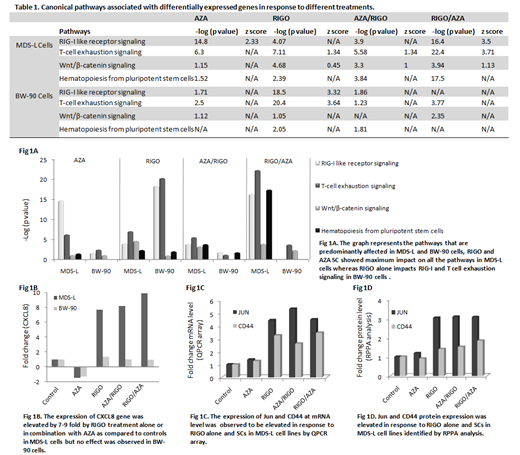
Results: We observed differential expression (DE) pattern of the genes in response to AZA, RIGO and their SCs both in MDS-L and BW-90 cell lines. The functional pathways associated with DE genes predominantly impacts RIG-I like receptor (RLR) signaling (anti-viral defense pathway), T cell exhaustion signaling, Wnt/β-catenin signaling and hematopoiesis pathway in MDS-L cells treated with RIGO/AZA combinations compared to other treatments (Table 1, Fig 1A). However, RIGO alone induces the dysregulation of RIG-I like receptor signaling and T cell exhaustion signaling in BW-90 cells (Table 1, Fig A). Expression of CXCL8 was observed to be elevated in RIGO and SCs by 7-9 fold compared to untreated MDS-L cells while no significant difference was found in response to any treatment in BW-90 cells (Fig 1B). CXCL8 is a RLR signaling responsive gene and is also one of the genes which were observed to be involved in hematopoiesis signaling identified by pathway enrichment analysis. Similar to QPCR data we observed striking differences in MDS-L and BW-90 cells in response to different treatments at protein level and functional pathways by RPPA analysis and IPA, respectively. RIGO is a RAS mimetic and interrupts RAS-RAF binding (Reddy et al ASH 2014), in this study we observed RIGO in combination with AZA inhibits several MAPK signaling pathways including PI3K/AKT/mTOR, ERK/MAPK and p38 MAPK signaling in MDS-L cells. RIGO had been reported to inhibit PI3K/AKT pathway as a single agent (Xu et al. Sci Rep 2014). In addition, Wnt/β-catenin pathway was predicted to be specifically activated in MDS-L cells with SCs. Both QPCR and RPPA results demonstrated activation of Wnt/β-catenin signaling pathway in response to RIGO alone and the combination with AZA. Importantly, expression of two genes Jun (proto oncogene) and CD44 (Wnt target gene) that are associated with the Wnt/β-catenin signaling pathway were upregulated at both mRNA (Fig 1C) and protein level (Fig 1D) which suggests a crucial role of RIGO in wnt signaling.
Conclusions: These data demonstrate that RIGO sequenced with AZA upregulates RLR, Wnt/β-catenin and hematopoiesis signaling. Involvement of CXCL8 (RLR responsive gene) and activation of wnt signaling genes suggests their linkage to hematopoiesis. Further studies are underway to determine the effects of these signaling pathways on improving hematopoiesis both in vitro and in vivo in the HMA clinical resistance setting to identify potential therapeutic targets to reverse bone marrow failure in pts with HMA resistance.
Navada:Onconova Therapeutics Inc: Research Funding. Reddy:Onconova Therapeutics Inc: Equity Ownership, Research Funding. Silverman:Medimmune: Research Funding; Onconova Therapeutics Inc: Patents & Royalties, Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal