Introduction
Allogeneic hematopoietic stem cell transplantation (AHSCT) is increasingly performed for older patients with AML; however, the optimal conditioning regimen for these patients remains unclear.
Methods: We retrospectively evaluated outcomes of 404 patients with AML, ≥60 years receiving AHSCT at our institution between 01/2005-08/2018 who received 4 conditioning regimens: 1) fludarabine+melphalan 100mg/m2 (FM100, N=78), 2) fludarabine+melphalan 140mg/m2 (FM140, N=89), 3) fludarabine+IV busulfan x 4 days with Bu AUC≥5,000/day (equivalent dose 130mg/m2/day) (Bu≥5,000, N=131), 4) fludarabine+IV busulfan x 4 days with Bu AUC 4,000/day (equivalent dose 110mg/m2/day) (Bu4,000, N=106). To adjust for potential selection bias in choices of conditioning regimen, propensity score was calculated and used as a stratifying variable in a multivariable Cox regression model. Factors included in the propensity score calculation were age, secondary AML, ELN2017 genetic risk, remission status before transplant, induction failure, donor type, stem cell source and KPS.
Results are presented for the FM100, FM140, Bu≥5,000 and Bu4000, respectively. Median follow-up survivors were 40, 74, 30 and 44 months, respectively (p=0.06). Donors are matched sibling, matched unrelated, haploidentical and mismatched unrelated donor in 126 (31%), 218 (54%), 40 (10%) and 20 (5%) patients, respectively. Patients in the FM100 group were significantly older and had lower KPS. The median age was 67, 64, 64 and 65 years, respectively (p=0.001), while 51%, 32%, 27% and 27% had KPS<90%, respectively (p<0.001). The HCT-CI of ≥3 was present in 57%, 62%, 56% and 70%, respectively (p=0.33), while 42%, 78%, 47% and 51% had high and very high-risk DRI, respectively (p<0.001), and 12%, 46%, 18% and 32% of the patients were transplanted in active disease (p<0.001). No significant differences were seen in both cytogenetic and ELN2017 genetic risk. More patients in FM100 group were treated using a standard of care protocol (73%, 64%, 25% and 31%, respectively, p<0.001).
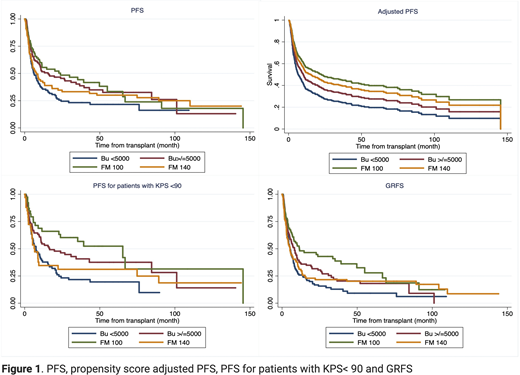
Grade 2-4 aGVHD at day 100 were 26% vs. 26%, 36% and 40% (p=0.04), and extensive cGVHD at 3 years 14% vs. 42%, 36% and 37%, respectively (p=0.07). The NRM at 3 years were 19%, 29%, 25% and 21% (p=0.06), and 3-year relapse rates were 32% vs. 32%, 30% and 55%, respectively (p=0.003). Among 4 groups, FM100 group had a significantly better PFS and GRFS with 5-year PFS for these 4 groups were 44%, 30%, 33% and 22% (p=0.02) and 5-year GRFS were 28%, 20%, 18% and 9% (p=0.006), respectively (Figure 1).
For subgroup of patients with KPS <90%, 5-year PFS were 41%, 27%, 28%, 22%, respectively (p=0.007), while there was no significant difference between 4 conditioning groups in patients with high-risk AML defined as either secondary AML, induction failure or high-risk cytogenetics/high ELN2017 risk, suggesting that a more intense conditioning is not beneficial in this group of patients.
The survival benefit of FM100 persisted after adjusted for baseline factors, transplant characteristics as well as propensity scores in a multivariable analysis (MVA). In MVA for PFS, HR was 0.57 (p=0.013) for FM100, 0.68 (p=0.056) for FM140 and 0.77 (p=0.137) for Bu> 5000 as compared with Bu 4,000 group (Figure 1). In the MVA for GRFS, HR for FM100, FM140 and Bu> 5000 was 0.53 (p=0.005), 0.78 (p=0.196), and 0.81 (p=0.178), respectively as compared with Bu 4,000 group.
Other factors that independently predicted PFS were secondary AML (HR 1.68, p=0.001), remission status before transplant (HR 1.82, p=0.048 for CR with MRD positive, HR 1.87, p=0.043 for CR with unknown MRD status and HR 2.86, p=0.001 for active disease at transplant as compared with CR with MRD negative), KPS (HR 0.98, p=0.005) and use of a mismatched unrelated donor (HR 2.46, p=0.001 compared with matched related donor transplant).
Conclusions: Older patients with AML benefit from a reduced-intensity conditioning with FM100 conditioning regimen, which was associated with better survival despite the fact that patients who could not receive more intense conditioning preferentially received this regimen. Higher intensity conditioning does not appear to improve survival in older patients. Alternative approaches to increase in conditioning intensity are needed to improve survival in patients with AML receiving allogeneic hematopoietic stem cell transplantation.
Ciurea:Kiadis Pharma: Membership on an entity's Board of Directors or advisory committees, Other: stock holder; Miltenyi: Research Funding; Spectrum: Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees. Bashir:Imbrium: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Membership on an entity's Board of Directors or advisory committees; Acrotech: Research Funding; StemLine: Research Funding; Spectrum: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Oran:Astex pharmaceuticals: Research Funding; AROG pharmaceuticals: Research Funding. Popat:Bayer: Research Funding; Incyte: Research Funding; Jazz: Consultancy. Konopleva:Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Ascentage: Research Funding; Calithera: Research Funding; Forty-Seven: Consultancy, Honoraria; Kisoji: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal