
Introduction
Myeloproliferative neoplasms (MPN) are associated with an increased rate of venous thromboembolism (VTE), which can be a major cause of morbidity and mortality especially in the more indolent diseases of polycythaemia rubra vera (PV) and essential thrombocythaemia (ET). Splanchnic vein thromboses are common in MPN and may have high recurrence rates between 15-20% over 10 years1. Traditionally, vitamin K antagonists (VKA) have been the mainstay of anticoagulation for MPN-associated VTE and recent data suggests a high rate of recurrent VTE on their discontinuation in MPN patients2. In the last 5 years, there has been a significant change to the use of direct oral anticoagulants (DOAC) in non-MPN associated VTE and limited data is available in the MPN setting due to the exclusion of malignancies from initial Phase III studies.
Methods
A retrospective review of patients treated at a single tertiary centre for MPN was performed. Clinical details were obtained from electronic clinical notes, imaging and prescriptions.
Results
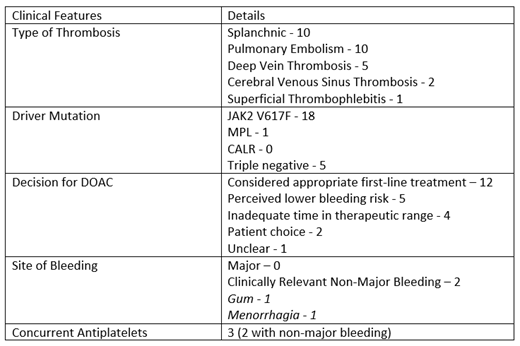
A total of 102 patients were identified as having a MPN-associated VTE with 24 of these receiving DOACs. The median age at VTE was 48 years (range 27.4-92.2 years) with 10 males (41.7%), primary diagnosis: 10 PV, 5 ET, 7 myelofibrosis (MF) and 2 MPN/myelodysplasia overlap. 18 patients were JAK2 V617F positive, 1 pt had MPL mutation and 5 pts were triple negative. In total 29 thrombotic events were recorded - 10 splanchnic (34.4%), 10 pulmonary embolism (34.4%), 5 deep vein thrombosis (17.2%), 2 cerebral venous sinus thrombosis (6.9%), 1 superficial thrombophlebitis (3.4%).
The median follow-up period was 2.2 years (range 0.7-7.5 years) with 16 patients initiated on DOAC (12 rivaroxaban and 4 apixaban) and 8 patients changed from VKA or heparin (3 rivaroxaban, 3 apixaban and 1 edoxaban). Two patients used more than one DOAC in this period. 21 patients were receiving long-term anticoagulation and 3 had defined courses of anticoagulation due to provocation. 21 patients were receiving cytoreductive agents or regular venesection along with anticoagulation.
During the total follow-up period of patients receiving DOACs, amounting to 66 pt years, there were no VTE recurrences in 23 patients and indeterminate imaging for 1. There were no episodes of major bleeding but 2 patients (8.3%) had clinically relevant non-major bleeding both of which were taking aspirin in addition to DOAC.
Conclusions
These results suggest that there were very low/no recurrence rates of VTE and no major bleeding in patients with MPN-associated VTE who are receiving DOACs. These include heterogeneous sites of VTE including splanchnic vein thromboses and cerebral venous sinus thrombosis. Of note, cytoreductive measures were also used in a majority of these patients. We suggest that DOACs could be safely used in this group in appropriately selected patients with MPN.
References:
1. Finazzi G, De Stefano V, Barbui T. Splanchnic vein thrombosis in myeloproliferative neoplasms: treatment algorithm. Blood Cancer J. 2018; 8(7): 64.
2. De Stefano V, Ruggeri M, Cervantes F et al. High rate of recurrent venous thromboembolism in patients with myeloproliferative neoplasms and effect of prophylaxis with vitamin K antagonists. Leukemia. 2016; 30(10): 2032-38.
McLornan:Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Novartis: Honoraria. Radia:Blueprint Medicines: Consultancy; Novartis: Consultancy, Speakers Bureau. Harrison:AOP: Honoraria; Promedior: Honoraria; Gilead: Speakers Bureau; Roche: Honoraria; Janssen: Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Sierra Oncology: Honoraria; CTI: Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Shire: Speakers Bureau; Incyte: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal