Background: Non-adherence to medications is recognized as one of the most important and costly worldwide healthcare problems in the 21st century; according to an EU report, non-adherence to therapies is responsible for 194,500 deaths and costs €125 billion annually. Taking into account those data, the use of adherence measurements in clinical trials could be extremely useful, in order to better understand patients (pts) behaviours and outcomes. The 8-item Morisky Medication Adherence Scale (MMAS-8, Morisky DE et al, J Clin Hypertens,2008 - Krousel-Wood MA et al, Am J Manag Care 2009- Morisky DE et al, J Clin Epidemiol. 2011) is a widely used questionnaire to asses indirectly pts adherence to medications. The first seven items are yes/no questions, and the last item is a five point Likert-scale. Ruxolitinib (RUX) is an oral JAK1/2 inhibitor approved for the treatment of symptoms and/or splenomegaly in myelofibrosis (MF) pts. ROMEI (CINC424AIT04-Ruxolitinib Observational study in Myelofibrosis treated patients in Italy) is a prospective observational study focused on real life management of MF pts with RUX; the trial enrolled 215 pts and included a prospective evaluation of MMAS-8 as a secondary endpoint. To date, no data regarding MMAS-8 and RUX are available and here we show a descriptive analysis of MMAS-8 after 24 weeks (wks) of RUX treatment.
Methods: After informed consent signature, pts started RUX according to Summary of Product Characteristics; MMAS-8 questionnaire was administered at wks 4, 8, 12 and 24 after RUX initiation. MMAS-8 were analysed only if pts provided all responses to the questionnaire; a score ˂ 6 indicates low adherence, a score between 6 and 8 indicates medium adherence, and a score = 8 suggests high adherence. As per protocol, only adherence classes (low-medium-high) assessment was scheduled and in case of low or medium adherence (ie suboptimal adherence), no action in term of target education or psychological interventions was planned.
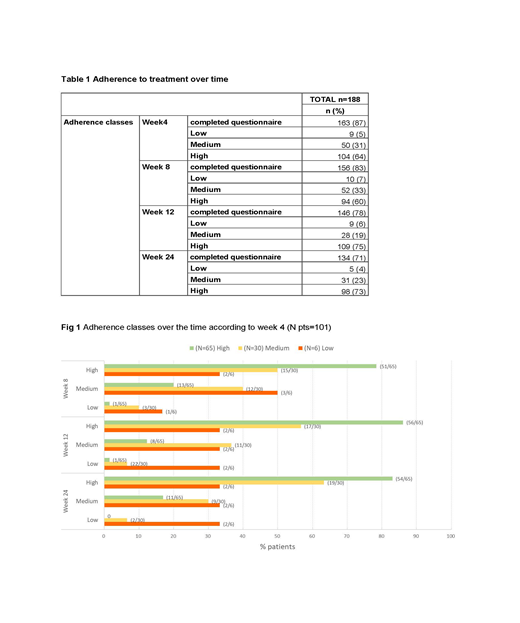
Results: Out of 215 enrolled pts, 188 were evaluable for MMAS-8. The MMAS-8 was completed by 163 pts (87%) at week (wk) 4, 156 pts (83%) at wk 8, 146 pts (78%) at wk 12 and 134 pts (71%) at wk 24. A total of 101 out of 188 eligible pts (54%) completed all 4 scheduled questionnaires. MMAS-8 total score seems to remain stable over the time. Mean MMAS-8 total score was 7.54±0.77 (min; max: 4.75; 8.00) at wk 4 and 7.67±0.70 (min; max: 4.25; 8.00) at wk 24, resulting in a mean change from wk 4 of 0.14±0.79 (min; max: -2.25; 2.25). Regarding adherence classes, the distribution between low, medium and high adherence over time is shown in table 1. Likewise, if we consider pts who completed all scheduled questionnaires, MMSA-8 total score remains stable over the wks, with total score of 7.53±0.79 (min; max: 4.75; 8.00) at wk 4, 7.60±0.71 (min; max: 4.75; 8.00) at wk 8, 7.69±0.69 (min; max 4.50; 8.00) at wk 12 and 7.67±0.73 (min; max: 4.25; 8.00) at wk 24, as well as adherence classes distribution (Fig 1). Regarding adherence classes in this subgroup of pts, seems that pts with high adherence at wk 4 are more prone to maintain the same class during time. Finally, we tried to perform an exploratory analysis relating spleen response according to IWG-MRT criteria with adherence classes at each time points: considering the small number of evaluable pts with complete information regarding both available parameters, no correlation between spleen responses and adherence classes was demonstrated (data available at the meeting).
Conclusion: This is the first time of MMAS-8 applied in MF pts as well as those treated with RUX. Our analysis showed a not irrelevant percentage of pts (ranging from 25% to 40%) belonging to a low-intermediate adherence classes during treatment. Surprisingly overall approximately one third of patients could be exposed to a suboptimal treatment over time and probably clinicians underestimate the number of those pts in clinical practice. Although we did not find in our cohort a correlation between adherence classes and spleen responses, probably due to low number of evaluable pts for both variables, the knowledge regarding pts attitude to therapy, especially for long-term and chronic treatments, remains crucial from clinical and pharmacoeconomic perspectives. Standardized methods to assess pts adherence such as MMAS-8 should be implemented in prospective well-designed clinical trials and could be a precious tool guiding clinicians in the everyday practice.
Palandri:Novartis: Consultancy, Honoraria. Cilloni:Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Palumbo:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Morelli:Novartis: Employment. Passamonti:Celgene Corporation: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Breccia:Bristol-Myers Squibb, Celgene, Incyte, Novartis, Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal