Introduction: Hydroxyurea (HU) is the recommended treatment in patients (pts) with polycythemia vera (PV) at high thrombotic risk. In 2013, European LeukemiaNet (ELN) guidelines defined criteria for response to HU including hematology and clinical parameters (Barosi G, Blood 2013). Yet, estimates of ELN response rates and of their influence on clinical outcomes are lacking.
Methods: The "PV-NET" is a European multicentre observational clinical study including now 530 cases of PV followed in 16 European Hematology Centers. Inclusion criteria are: 2016 WHO diagnosis of PV; availability of clinical/laboratory data at diagnosis and during follow-up; age≥18 yrs. Data cut-off was June 2019. A time-to-event (thrombosis, hemorrhage, evolution into blast phase [BP] or myelofibrosis [MF]) analysis was calculated from HU start with Fine & Gray model with death as competing risk. Overall survival (OS) was calculated from HU start to last contact/death (log-rank p). Response to HU was defined per ELN criteria: Complete (CR): Hematocrit (Hct) <45% without phlebotomies (PHL) & PLT ≤400×109/L & WBC ≤10×109/L & normal spleen size & no PV-related symptoms; Partial (PR): Hct <45% without PHL or response in 3/4 criteria.
Results: Overall, 438 required HU and were observed for 3069 pt-yrs. Characteristics at diagnosis were: median age: 62.3 yrs (22.3-89.5); males: 52.5%; median (range) WBC/PLT count, x109/l: 10.8 (1.1-33)/490 (143-1070); median hemoglobin (g/dl)/Hct (%): 18.6/56 (males); 17.6/54.7 (females); 56 (12.8%) and 30 (6.9%) pts had a thrombosis prior to or at diagnosis, respectively. Overall, 327 (74.7%) pts reported at least one PV-related symptom and 166 pts (37.9%) had a palpable spleen (≥10 cm: 8.4%).
Median time from diagnosis to HU start was 2.9 (0.07-238) mos. At HU start, 350 (79.9%) pts were at high thrombotic risk. HU was used first-line in 426 (97.3%) pts and second-line in 12 pts (10 pts after interferon). Median HU dose was 0.5 g/d (0.25-2); 21.7% of pts received ≥ 1 g/d.
After HU start, 36 pts presented 50 all-grades thromboses (arterial: 50%; grade ≥3: 51.1%), for an incidence rate of 1.6 per 100 pt-yrs (grade ≥3: 0.7). Thromboses were: deep/superficial vein thrombosis (32%/10%), acute myocardial infarction (12%), stroke (10%), transient ischemic attack (12%), spleen infarction (12%), retinal artery occlusion (4%), pulmonary embolisms (6%), and one splanchnic vein thrombosis. Thirteen bleedings (gastrointestinal: 61.5%; mucocutaneous: 30.8%; one hemothorax) occurred in 11 pts, for an incidence rate of 0.5 (grade ≥3: 69.2%). Overall, 12 progressions to BP and 29 MF evolutions were recorded (incidence rates: 0.5 and 1.3 X 100 pt-yr); 31 pts died.
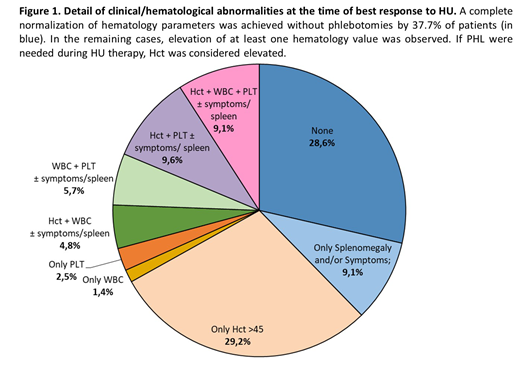
At the time of best response to HU, 62.3% of pts had at least one elevated hematology value; 231 (52.7%) pts continued PHL during HU (median PHL per yr: 2.5 [1-4]) (Fig.1). Per ELN criteria, 125 pts (28.6%) and 228 (52%) achieved a CR and PR, respectively, while 85 (19.4%) had no response (NR). The type of ELN response to HU (CR vs PR vs NR) did not affect the probability of thrombosis (p=0.56), hemorrhages (p=0.70), evolution to BP (p=0.60) or MF (p=0.14), and OS (p=0.37).
After a median follow-up from HU start of 4.2 yrs, 95 (21.7%) patients discontinued HU. The percentage of pts who discontinued HU was 8.4%, 16.2% and 19.4% at 5, 10 and 15 yrs and was significantly lower in CR pts compared to pts with PR and NR (p=0.02). The overall HU discontinuation rate was 4.1 per 100 pt-yr. Reasons for HU discontinuations were: failure to control hct and/or leucocytosis and/or thrombocytosis (16.9%), failure to reduce splenomegaly and/or symptoms (7.4%), MF (12.6%) or BP (4.2%) evolution, second neoplasia (4.2%). A total of 52 pts (54.7%) discontinued due to HU-related toxicity, specifically: skin lesions (46.3%), oral aftosis (14.6%), gastrointestinal disorders (12.2%), fever (9.8%), thrombocytopenia (9.8%), and anemia (7.3%). Survival was not influenced by HU discontinuation (p=0.50).
Conclusions: ELN-defined CR was rarely achieved by HU-treated pts, mainly due to low HU doses and PHL requirement, and did not influence outcome parameters. These data outline the relatively low utility in the current clinical practice of ELN criteria for the evaluation of HU treatment; their implementation would be relevant to base therapy changes on prognostic considerations. Finally, around 20% of pts discontinued HU, confirming that there is room for improvement in PV treatment strategy.
Palandri:Novartis: Consultancy, Honoraria. Elli:Novartis: Membership on an entity's Board of Directors or advisory committees. Benevolo:Novartis Pharmaceuticals: Consultancy. Tiribelli:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Bonifacio:Novartis: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; BMS: Honoraria. Cavazzini:Pfize: Honoraria; Incyte: Honoraria; Novartis: Honoraria. Palumbo:Novartis: Honoraria; Teva: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Hospira: Honoraria. Heidel:Novartis: Consultancy, Research Funding; Celgene: Consultancy; CTI: Consultancy. Crugnola:Incyte: Honoraria; Novartis: Honoraria. Cuneo:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Krampera:Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Breccia:Novartis: Honoraria; Celgene: Honoraria; BMS: Honoraria; Pfizer: Honoraria; Incyte: Honoraria. Cavo:sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; novartis: Honoraria; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; bms: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Latagliata:Celgene: Honoraria; Pfizer: Honoraria; Janssen: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal