Introduction: Glivec, Imatinib (IM), first tyrosine kinase inhibitor (TKI) has proven its efficacy and safety in Chronic Myeloid Leukemia - Chronic Phase (CML CP) in a large number of trials. In developing country like India Generic Imatinib formulations have been used recently as a more cost effective treatment, data on efficacy and safety of these drugs is sparse.
Aim: To analyse and compare safety profile, the overall survival (OS) and progression-free survival (PFS) data of CML-CP patients receiving Glivec/Generic IM as first line therapy
Method: A single centre retrospective analysis done on CML-CP patients who initiated treatment with either Glivec or Generic IM between July 2012 and July 2019 at Hemato-oncology Care Centre (HOCC) Vadodara India. Total 109 newly diagnosed CML-CP received IM as 400mg daily dose, dose was adjusted if significant cytopenia observed or in paediatric patients. Response assessment was made as per NCCN CML Version 1 2019 guidelines.Molecular monitoring of patients was done through BCR-ABL1 RQPCR, reported on international scale. Major Molecular Response (MMR) was considered when the ratio on international scale was less than 0.1% level or ≥ 3-log reduction in BCR-ABL1 mRNA from the standardized baseline, while Deep Molecular Response-MR4 was considered when the ratio on international scale was less than 0.01%.OS was measured from starting date of IM until to the date of death from any cause while on therapy or last seen.PFS was measured from starting date of IM to transformation to AP or BC or deaths while on therapy. Survival curves were calculated by the Kaplan-Meier method and compared with the log-rank test. Cox regression with Backward Wald Method, Chi-square test and t-test is applied using SPSS software version 21.0. A p-value <0.05 is considered to be statistically significant.
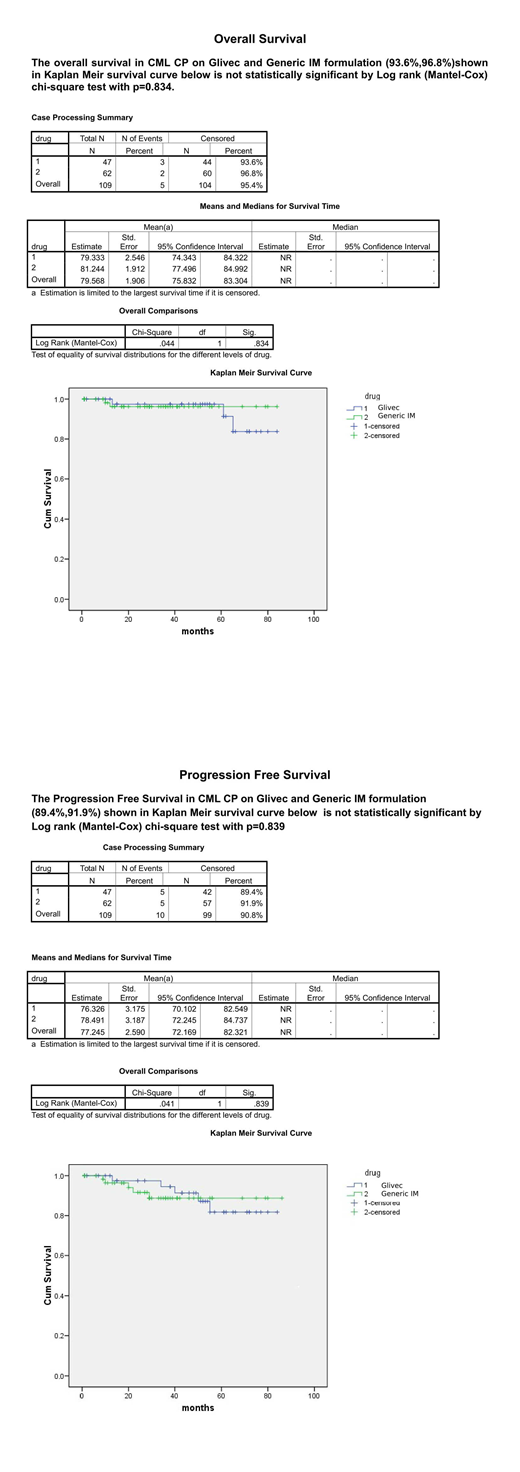
Results: A Total 109 patients were analysed, received Glivec (47)43% or Generic IM (62)56%. The mean (SD) age was 42.2 years (13.3 years) in Glivec and 47.5 years (14.9 years) in the Generic IM group (P=0.06). There was male preponderance in both groups (Glivec 55%, Generic IM 62 %). Sokal score stratification in Glivec and Generic groups, was respectively: low risk 43%/40%; intermediate risk 36%/48% and high risk 21%/11% (P=0.08). The median follow‐up period was 36 months (range, 9-84). MMR was 61% and 38% in Glivec and Generic IM respectively(p=0.02), MMR was achieved in 40%,04%,11%,06% of Glivec and 29%,2%,5%,2% of Generic IM group patients, at 1st,2nd,3rd,4th year of treatment respectively(P=0.15).Deep Molecular Response-MR4 was significantly higher 40% in Glivec vs 23% in Generic IM group (P=0.01). Nearly 35% of patients were lost to follow up, 28% and 40% in Glivec and Generic IM group respectively(p=0.27). Reasons for drop out were - cost constraints, noncompliance, lack of understanding, lack of transport. Dose limiting cytopenia was observed in initial 3 months of treatment in 12% and 23 % in Glivec and Generic IM group respectively(P=0.28).The most common non haematological side effects in Glivec and Generic groups were skin hyperpigmentation 19%,17%, abdominal discomfort 16 %,11% ,Facial Puffiness 9%,5%, respectively(P =0.73). In each group 4% patients progressed to AP, managed with second generation TKI in Generic IM group and with increasing dose of Glivec to 600mg daily in Glivec group. Total 5% patients progressed to BC and died, 3% in Glivec vs 2% in Generic IM group. The risk factors for progression to AP or BC were high Sokal score, poor molecular response and dose limiting cytopenia. OS and PFS at 36 months were similar in Glivec and Generic IM (93.6% vs. 96.8% P=0.834) and (89.4% vs. 91.9%, P=0.839) respectively.
Conclusion: Both the groups have shown similar OS and PFS at 36 months of median follow up, there was also no difference in the safety profile, meeting Generic IM unmet needs in developing country. However in view of higher MMR and Deep molecular response-MR4 in Glivec group, need long term follow up to determine its impact on outcome especially in reference to treatment free remission.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal