Background: Bosutinib, a Src/Abl tyrosine kinase inhibitor, is approved at a starting dose of 500 mg once daily (QD) in many countries, including Japan, for patients with Philadelphia chromosome-positive (Ph+) chronic phase (CP), accelerated phase (AP), or blast phase (BP) chronic myeloid leukemia (CML) after prior therapy. The indication for bosutinib was expanded to patients with newly diagnosed CP CML, at a starting dose of 400 mg QD, in 2017 by the US Food and Drug Administration and in 2018 by the European Medicines Agency. Approval of first-line bosutinib for CP CML was based on data from the global phase 3 BFORE trial, which demonstrated a significantly higher major molecular response (MMR) rate at Month 12 with bosutinib vs imatinib in patients with Ph+ CP CML and e13a2/e14a2 transcripts (primary endpoint; 47.2% vs 36.9%; 2-sided P=0.02). We conducted a phase 2 study to evaluate the efficacy, safety, and pharmacokinetics (PK) of bosutinib in Japanese patients with newly diagnosed CP CML.
Methods: In this open-label, single-arm study (NCT03128411), Japanese patients ≥20 years of age with a molecular diagnosis of CP CML within 6 months, Eastern Cooperative Oncology Group performance status 0 or 1, adequate renal and hepatic function, and no prior treatment for CML (hydroxyurea within 6 months permitted) received bosutinib at a starting dose of 400 mg QD. The primary endpoint was MMR at Month 12 in the modified as-treated population, which included patients who were Ph+ and had e13a2/e14a2 transcripts. A total of 60 patients was required in the modified as-treated population for the study to have >82% power to reject the null hypothesis (25% MMR rate at Month 12) and accept the alternative hypothesis (40% MMR rate at Month 12) with a 1-sided ∝-level of 5%. Secondary endpoints included MMR and complete cytogenetic response (CCyR) by Month 12, event-free survival (EFS), overall survival, safety, and PK.
Results: In all, 60 Japanese patients with CP CML were treated with bosutinib; all patients were Ph+ and had e13a2/e14a2 transcripts and were included in the modified as-treated population analyzed for efficacy. Median age was 55 years (range 20-83), 60.0% of patients were male, and 45.0%, 43.3%, and 11.7% had low-, intermediate-, and high-risk Sokal scores, respectively. Median duration of follow-up was 16.6 months (range 11.1-21.9), and median duration of bosutinib treatment was 15.3 months (range 0.3-21.9). After 12 months of follow-up, 42 (70.0%) patients remained on bosutinib; 17 (28.3%) discontinued due to adverse events (AEs) and 1 (1.7%) due to physician decision. Median dose intensity was 354.7 mg/day (range 95.3-494.1).
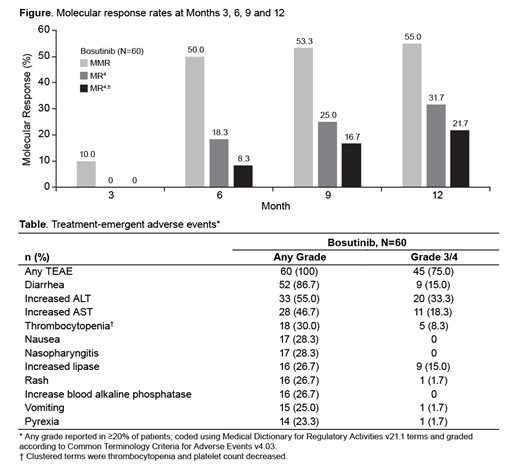
The MMR rate at Month 12 was 55.0% (2-sided 90% confidence interval [CI] 44.4-65.6); the test of the null hypothesis was rejected (1-sided P<0.0001), and the primary endpoint was met. The MMR rate by Month 12 was 61.7% (90% CI 51.3-72.0), and the CCyR rate by Month 12 was 80.0% (90% CI 71.5-88.5). Deep molecular responses (MR4/MR4.5) were achieved at Months 6, 9, and 12 (Figure). The cumulative incidence of EFS events at Month 12 was 1.7% (90% CI 0.2-6.4). There were no on-treatment transformations to AP/BP CML. No patient died on treatment or within 28 days of the last dose of bosutinib; 1 patient died beyond 28 days of the last dose due to disease progression.
The most common treatment-emergent AEs (TEAEs) were diarrhea (86.7%), increased alanine aminotransferase (ALT; 55.0%) and increased aspartate aminotransferase (AST; 46.7%; Table). Grade 3/4 TEAEs reported in ≥10% of patients were increased ALT (33.3%), increased AST (18.3%), diarrhea (15.0%), increased lipase (15.0%), and neutropenia (11.7%). The incidence of cardiac, vascular, and hypertension TEAEs was low (5.0%, 1.7%, and 1.7%, respectively).
The average bosutinib plasma trough concentration (mean ± standard deviation of concentrations on Days 28, 56, and 84) was 82.7 ± 48.0 ng/mL, ~1.12-fold higher than that observed in bosutinib-treated patients in the global BFORE trial.
Conclusions: The primary objective of this phase 2 study was met, and the MMR rate at Month 12 in Japanese patients with newly diagnosed CP CML was similar to that reported in the bosutinib arm of the multinational BFORE trial. The safety profile of bosutinib was consistent with previous studies. These data suggest bosutinib is an effective first-line treatment option for Japanese patients with newly diagnosed CP CML.
Takahashi:Novartis Pharmaceuticals: Research Funding, Speakers Bureau; Eisai Pharmaceuticals: Research Funding; Chug Pharmaceuticals: Research Funding; Pfizer: Research Funding, Speakers Bureau; Ono Pharmaceutical: Research Funding; Astellas Pharma: Research Funding; Asahi Kasei Pharma: Research Funding; Otsuka Pharmaceutical: Research Funding, Speakers Bureau; Kyowa Hakko Kirin: Research Funding; Bristol-Myers Squibb: Speakers Bureau. Matsumura:Novartis: Speakers Bureau; Otsuka Pharmaceutical: Consultancy, Research Funding; Pfizer: Research Funding, Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau. Ishizawa:Otsuka Pharmaceutical: Research Funding; Pfizer: Research Funding; Novartis: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau. ONO:Otsuka Pharmaceutical Co., Ltd.: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Honoraria; Chugai Pharmaceutical Co.,Ltd.: Research Funding; Kyowa Hakko Kirin: Research Funding; ONO Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Merck Sharp & Dohme: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Takeda Pharmaceutical Co., Ltd.: Honoraria. Sekiguchi:Pfizer: Research Funding. Tanetsugu:Pfizer R&D Japan G.K.: Employment. Fukuhara:Pfizer R&D Japan G.K.: Employment. Ohkura:Pfizer R&D Japan G.K.: Employment. Koide:Pfizer R&D Japan G.K.: Employment. Hino:Alexion: Honoraria; Astellas Pharma Inc: Honoraria, Research Funding; Astellas Amgen BioPharma: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Chugai Pharmaceutical Co., Ltd: Honoraria, Research Funding; Daichi-Sankyo: Honoraria, Research Funding; Eisai: Research Funding; Janssen: Honoraria; Japan Blood Products Organization: Honoraria, Research Funding; Kyowa-Hakko Kirin Co.,Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Consulting fee, Research Funding; Mochica Pharmaceutical Co., Ltd: Honoraria; MSD: Honoraria, Research Funding; Mundipharma: Honoraria; Nihon Pharmaceutical Co., Ltd: Research Funding; Nippon Shinyaku: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceutical: Honoraria, Other: Consulting fee, Research Funding; Otsuka Pharmaceutical: Honoraria, Research Funding; Pfizer Japan Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Honoraria; Shire Japan KK: Honoraria; Sumitomo Dainippon Parma: Honoraria, Research Funding; Taiho Pharama: Research Funding; Takeda Pharmaceutical Co., Ltd: Honoraria, Research Funding; Teijin: Research Funding; Abbott: Research Funding.
Bosutinib is approved for newly diagnosed patients with chronic phase chronic myeloid leukemia by the US Food and Drug Administration and by the European Medicines Agency, but it is not yet approved for this patient population in Japan, where this study takes place.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal