Introduction: Early responses to tyrosine kinase inhibitors (TKIs) are associated with improved long-term outcomes in patients with chronic myeloid leukemia in chronic phase (CML-CP), and guideline recommendations support the achievement of major molecular response (MMR) at 18 months as a therapeutic goal in CML treatment. Dasatinib is a first-line (1L) treatment option for patients with CML-CP, and long-term results from the DASISION study have demonstrated that patients on dasatinib achieved faster, deeper, and more durable molecular responses than patients on imatinib (Cortes J et al. J Clin Oncol 2016). Earlier reports have shown that obesity may increase the risk of developing CML (Strom SS et al. Cancer Epidemiol Biomarkers Prev 2009) and that patients with a high body mass index (BMI; > 25 kg/m2) at diagnosis who receive 1L imatinib have a significantly longer median time to response and a reduced rate of MMR compared with patients with a normal BMI (< 18.5-25 kg/m2; Breccia M et al. Cancer Lett 2013). In this exploratory post hoc analysis of the phase 3 DASISION trial (NCT00481247), we further investigated the association of high BMI with treatment responses with 1L TKIs.
Methods: DASISION was a multinational, open-label, phase 3 trial of dasatinib versus imatinib for newly diagnosed CML-CP. Patients were randomized to receive 100 mg dasatinib (n = 259) or 400 mg imatinib (n = 260) once daily. Response outcomes were retrospectively stratified on the basis of two BMI categories: high (≥ 25 kg/m2) and normal (< 25 kg/m2). Median time to response was estimated using Kaplan-Meier analysis; Cox proportional hazard models and log-rank tests were stratified by Hasford scores. Molecular response rates were compared using Cochran-Mantel-Haenszel tests (stratified by Hasford scores). P values are descriptive and unadjusted for multiple comparisons.
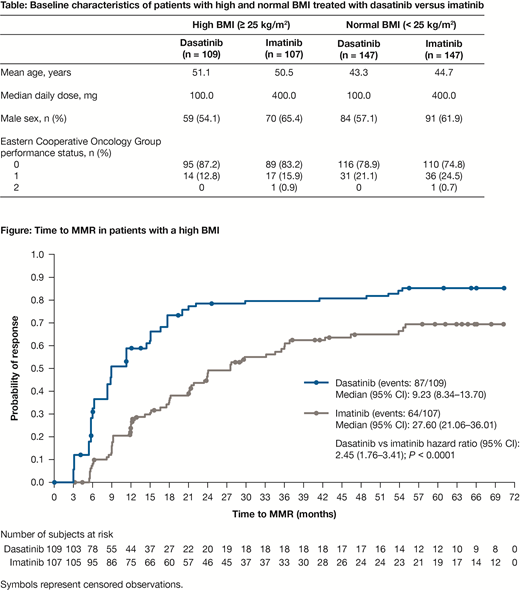
Results: In total, 109 patients with a high BMI and 147 patients with a normal BMI were treated with dasatinib, and 107 patients with a high BMI and 147 patients with a normal BMI were treated with imatinib. Baseline characteristics were balanced within BMI subgroups and are listed in the table below (Table). Median time to complete cytogenetic response (CCyR) was significantly shorter with dasatinib versus imatinib in patients with a high BMI (3.1 vs 6.1 months; P < 0.0001). MMR was also achieved faster in patients with a high BMI who were treated with dasatinib versus imatinib (median time 9.2 vs 27.6 months; P < 0.0001; Figure). More patients with a high BMI treated with dasatinib achieved MMR compared with those treated with imatinib (79.8% vs 59.8%; P = 0.0004). Likewise, 54.1% of patients with a high BMI achieved MR4.5 with dasatinib, compared with 34.6% with imatinib (P = 0.0013). In the normal BMI group, median time to CCyR (5.6 vs 6.0 months; P = 0.1055) and MMR (18.0 vs 21.5 months; P = 0.4095) was faster for dasatinib versus imatinib, and more patients on dasatinib versus imatinib achieved MMR (73.5% vs 67.3%; P = 0.3335) and MR4.5 (36.7% vs 33.3%; P = 0.6344). Although these results were numerically better with dasatinib, the differences were not statistically different. A graphical exploratory analysis suggested that there was no difference in exposures across BMI subgroups with respect to dasatinib. However, imatinib exposure data were not available to make comparisons across the BMI subgroups. There was no major difference in the previously reported adverse event profiles between treatment groups when assessed based on BMI. Any-cause pleural effusion occurred more frequently with dasatinib (34.3% [high BMI] and 24.5% [normal BMI]) compared with imatinib (0% [high BMI] and 2.0% [normal BMI]). Additional analyses are being planned to address the role of any potential confounders (eg, Hasford risk scores).
Conclusions: In this exploratory post hoc analysis, patients with a high BMI treated with dasatinib demonstrated a significantly faster time to response compared with imatinib, with an increased percentage of patients also achieving MMR and MR4.5 at 5 years. However, these differences were not apparent in patients with a normal BMI. Although these findings highlight the potential role of BMI in affecting treatment responses to TKIs, additional validation of these findings is necessary to define the overall impact of BMI as a prognostic factor for patients with CML-CP.
Study support: BMS. Writing support: Jane Cheung, Caudex, funded by BMS.
Breccia:Bristol-Myers Squibb, Celgene, Incyte, Novartis, Pfizer: Honoraria. Cortes:Bristol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Sun Pharma: Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; BiolineRx: Consultancy. Shah:Bristol-Myers Squibb: Research Funding. Saglio:Celgene: Consultancy; Jansen: Consultancy; Pfizer: Consultancy; BMS: Consultancy; Novartis: Consultancy; Ariad: Consultancy; Incyte: Consultancy. Le Coutre:Novartis: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau. Brun:Bristol-Myers Squibb: Employment. DeGutis:Bristol-Myers Squibb: Employment, Other: Stock options. Sy:Bristol-Myers Squibb: Employment, Equity Ownership. Jabbour:AbbVie: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal