Background: The clinical course of sickle cell disease (SCD) varies greatly by patient depending on age, complications, comorbidities, and psychosocial health. While researchers have developed models to predict complications and mortality, there is currently no accepted classification system of overall SCD severity. Our goal was to develop a severity classification system that could be tested for its ability to predict clinical outcomes.
Methods: Using a RAND/UCLA modified Delphi panel, a method that has content, construct, and predictive validity, we convened 10 expert clinicians (9 MDs, 1 DNP) from various backgrounds (5 hematologist/oncologists, 3 internists, 1 psychiatrist/public health practitioner, 1 pulmonologist) with an average of 20 years of experience caring for SCD patients. Experts were provided with a review of evidence drawn from the 2014 National Heart, Lung, and Blood Institute Expert Panel Report, focusing on factors associated with morbidity and mortality in SCD. They collaboratively developed then rated 180 patient scenarios with varying characteristics (age, HbSS/HbSβ0/HbSC/HbSβ+ genotype, no/mild or moderate/severe end organ damage, presence/absence of chronic pain, and number of unscheduled acute care visits per year due to vaso-occlusive crises (VOCs)) on multiple axes including risk of complications or death, quality of life impact, and overall disease severity using a 1 to 9 scale. Each scenario was a simplified patient history in which end organ damage was defined as either severe damage to organs fed by the circulatory system (e.g., congestive heart failure, ≥Stage 3 kidney disease, overt stroke); mild/moderate damage (e.g., hypoxia, Stage 1 or 2 kidney disease, transient ischemic attack in the absence of stroke); or no damage. Other characteristics were also categorized in a simplified fashion (e.g., present/absent chronic pain defined as ongoing pain on most days over the past 6 months). Ratings were completed independently by each expert before an all-day in-person meeting. Areas of disagreement were discussed. Median ratings were grouped into 3 categories (1-3, 4-6, 7-9) and disagreement was defined as ≥2 individual ratings outside the median category. At the conclusion, experts completed ratings again. These final round ratings were used to develop a 3-level severity classification system ranging from Class I (least severe) to Class III (most severe).
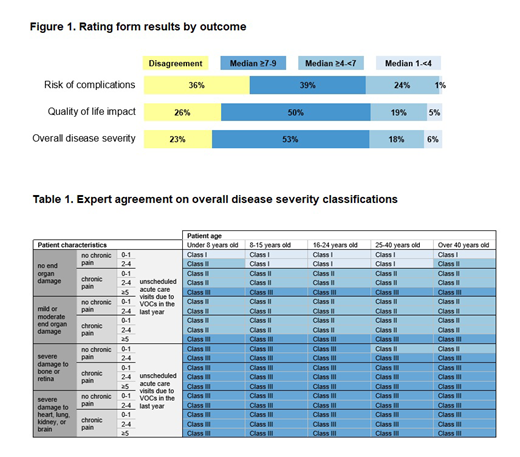
Results: The proportion of items with disagreement decreased from 64% to 32% following the meeting. In the final round, experts agreed on the overall disease severity of 77% of scenarios (Figure 1). Scenarios that differed only by hemoglobin genotype were rated similarly, with differences in the 0.0-0.4 range out of 9. Using final round ratings as a guide, experts agreed patients 8-40 years old with no end organ damage, no chronic pain, and ≤4 unscheduled acute care visits due to VOCs in the last year should be classified as Class I (least severe disease). Patients <8 or >40 years old with no end organ damage, no chronic pain, and <2 unscheduled acute care visits due to VOCs in the last year were also considered Class I. Patients any age with ≥5 unscheduled acute care visits due to VOCs in the last year were considered Class III (most severe disease). Similarly, patients any age with severe damage to bone, retina, heart, lung, kidney, or brain were classified as Class III, with the exception of patients ≥25 years old presenting with severe retinopathy, no chronic pain, and 0-1 unscheduled acute care visits due to VOCs in the last year, who were considered Class II. All other patients were classified as Class II (Table 1). Experts agreed that genotype does not affect classification.
Conclusion: A validated methodology was used to assist a multi-disciplinary expert panel in developing a classification system for SCD severity, which could be implemented in a clinical setting. The system differs from earlier prediction models of disease severity: Instead of estimating mortality risk using mathematical modeling, it consolidates patient characteristics into homogenous groups of patients with respect to disease state to support clinical decision-making. The system is consistent with existing literature that increased unscheduled acute care visits and organ damage translate into clinically significant patient morbidity. Studies to further validate this system using patient reported and clinical outcomes are planned.
Shah:GBT: Research Funding; Alexion: Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau. Beenhouwer:Partnership for Health Analytic Research (PHAR), LLC: Other: I am an employee of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by Novartis to conduct the research described in this abstract.. Broder:Partnership for Health Analytic Research (PHAR), LLC: Other: I am an employee of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by Novartis to conduct the research described in this abstract.. Bronte-Hall:Novartis: Consultancy. De Castro:Pfizer: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Gibbs:Partnership for Health Analytic Research (PHAR), LLC: Other: I am an employee of the Partnership for Health Analytic Research (PHAR) LLC, which was paid by Novartis to conduct the research described in this abstract.. Gordeuk:CSL Behring: Consultancy, Honoraria, Research Funding; Emmaus: Consultancy, Honoraria; Global Blood Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Modus Therapeutics: Consultancy, Honoraria; Pfizer: Research Funding; Inctye: Research Funding; Ironwood: Research Funding; Imara: Research Funding. Kanter:Rockpointe: Honoraria; Medscape: Honoraria; Sangamo: Consultancy; GLG: Consultancy; SCDAA: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria; Imara: Consultancy; Jeffries: Consultancy; bluebird bio, Inc: Consultancy; NHLBI: Membership on an entity's Board of Directors or advisory committees; Guidepoint Global: Consultancy; Cowen: Consultancy; Modus: Consultancy; Peerview: Honoraria. Lipato:Novartis: Honoraria. Manwani:GBT: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Consultancy. Scullin:Novartis: Consultancy, Honoraria. Yermilov:Partnership for Health Analytic Research (PHAR), LLC: Other: I am an employee of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by Novartis to conduct the research described in this abstract.. Smith:Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal