Clinical resistance to tyrosine kinase inhibitors (TKI) remains a significant problem in the therapy of patients with chronic myeloid leukemia (CML). Although BCR-ABL1-kinase domain mutations are the major cause of resistance to TKI, some patients manifest primary or develop secondary resistance to TKI without detectable BCR-ABL1 mutations. We aimed to assess the prevalence of additional gene mutations in a group of 50 patients with primary (n = 26) or secondary resistance (n = 24) to TKI, in most cases to imatinib (n = 49) and in one to dasatinib. We employed ~1000 genes custom target enrichment kit and next-generation sequencing on Illumina platform, as well as Sanger sequencing. In 21 patients, we were able to match and analyze paired samples collected at diagnosis (before treatment) and at the time of resistance and in five other cases we analyzed additional samples collected later during TKI-resistant chronic phase or in blast crisis.
The most frequent genetic aberrations detected at the time of TKI-resistance were mutations in ASXL1 and BCR-ABL1 kinase domain coding sequence, present in 28% (14/50) and 26% (13/50) of patients, respectively. Both genes were mutated in 12% of patients (6/50), while 30% (15/50) had either ASXL1 or BCR-ABL1 mutations alone. Non-recurrent genetic aberrations in other genes were also noted in single patients (e.g. DNMT3A mutations).
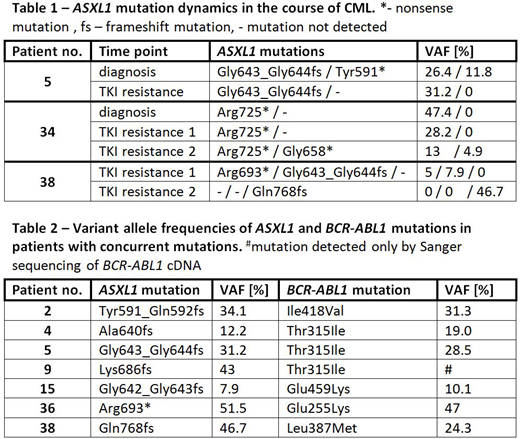
According to COSMIC database, all but three ASXL1 mutations detected in this study were previously described in hematologic malignancies and all (including the novel ones) are predicted to introduce stop codons or cause frameshifts in codon range of 512-943, thus truncating the protein before the C-terminal PHD domain. Among patients with available paired samples three had more than one ASXL1 mutation at various time points and in all of them we observed dynamics of specific ASXL1 mutations, related partially to the leukemic cell content in the sample but also providing evidence of clonal evolution (Table 1). In most of patients with concurrent ASXL1 and BCR-ABL1 mutations, we observed that both mutations were present with similar variant allele frequency (VAF), suggesting that ASXL1 mutations occur in Philadelphia-positive leukemic clones (Table 2).
ASXL1 mutation frequency was significantly higher in TKI-resistant patients, than in our previously characterized group of CML patients without resistance, who achieved major molecular response (28% ; 14/50 vs 5.6% ; 2/36, respectively, p = 0.0105, Fisher exact test). All ASXL1-mutated patients with secondary (n = 1) or primary resistance (n = 5) for whom diagnostic sample was available, carried ASXL1 mutation also at the time of diagnosis. No significant difference in mutation frequency was found between patients with primary and secondary resistance (ASXL1 mutation, 26.9% vs 29.2% ; BCR-ABL1 mutation, 15.4% vs 37.5%).
Our results provide evidence that preexisting ASXL1 mutations in BCR-ABL1-positive leukemic clone present at diagnosis may have impact on clinical response to imatinib and may be useful in assessing the risk of treatment failure.
Niesiobedzka-Krezel:Novartis: Honoraria. Seferynska:Novartis: Honoraria. Gora Tybor:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sacha:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal